Abstract
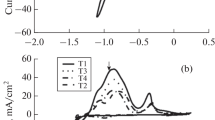
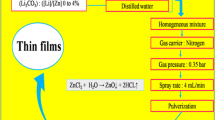
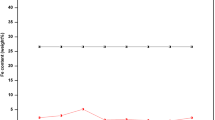
Nine distinct zinc-nickel-tin films with different compositions have been galvanostatically electrodeposited. The films have been characterized by scanning electron microscopy (SEM) and energy dispersive spectrometry (EDS). Their corrosion potentials and densities have been estimated using Tafel extrapolation. Next, the electrochemical behaviors of the films (deposited through the electrolytes containing 0, 6, 8, and 10 g/L SnCl2∙6H2O) have been examined based on cyclic voltammetry (CV) measurements. Further, these films have been immersed in 3.5wt% NaCl solution for 1 h, 1 d, 7 d, 14 d, 28 d, and 42 d followed by application of Tafel extrapolation and electrochemical impedance spectroscopy (EIS) tests on each aged sample. Finally, to analyze the morphologies and the compositions of the oxide films covering the surfaces of the 42-d aged films, FT-IR and SEM analyses have been performed. The results indicated that the Zn–Ni–Sn film produced through the bath including 6 g/L SnCl2∙6H2O exhibits superior corrosion resistance because of the high Ni content in the presence of Sn that promotes the barrier protection capability of the deposit.
Similar content being viewed by others
References
[1] A. Brenner, Electrodeposition of Alloys, Academic Press, New York and London, 1963.
[2] H. Fukushima, T. Akiyama, M. Yano, T. Ishikawa, and R. Kammel, Electrodeposition behavior of Zn-iron-group metal alloys from sulfate and chloride baths, ISIJ Int. 33(1993), No. 9, p. 1009.
[3] Y.P. Lin and J.R. Selman, Electrodeposition of corrosion-resistant Ni-Zn alloy, J. Electrochem. Soc, 140(1993), No. 5, p. 1299.
[4] G. Roventi, T. Bellezze, and R. Fratesi, Electrochemical study on the inhibitory effect of the underpotential deposition of zinc on Zn-Co alloy electrodeposition, Eec trochim. Acta, 51(2006), No. 13, p. 2691.
[5] Z.D. Wu, L. Fedrizzi, and P.L. Bonora, Electrochemical studies of zinc-nickel codeposition in chloride baths, Surf. Coat. Technol, 85(1996), No. 3, p. 170.
[6] T.V. Byk, T.V. Gaevskaya, and L.S. Tsybulskaya, Effect of electrodeposition conditions on the composition, mcro-structure, and corrosion resistance of Zn-Ni alloy coatings, Surf. Coat. Technol., 202(2008), No. 24, p. 5817.
[7] F.J. Miranda, I.C.P. Margarit, O.R. Mattos, O.E. Barcia, and R. Wiart, Corrosion behavior of zinc-nickel alloy elec-trodeposited coatings, Corr. Sci, 55(1999), No. 8, p. 732.
[8] XG. Zhang, Galvanic corrosion of zinc and its alloys, J. Electrochem. Soc, 143(1996), No. 4, p. 1472.
[9] K. Wang, H.W. Pickering, and K.G. Weil, EQCM studies of the electrodeposition and corrosion of tin-zinc coatings, Electrochim. Acta, 46(2001), No. 24–25, p. 3835.
[10] EA. Pavlatou, M. Stroumbouli, P. Gyftou, and N. Spyrellis, Hardening effect induced by incorporation of SiC particles in nickel electrodeposits, J. Appl. Electrochem. 36(2006), No. 4, p. 385.
[11] A. Durairajan, A. Krishniyer, B.S. Haran, R.E. White, and B.N. Popov, Characterization of hydrogen permeation through a corrosion-resistant zinc-nickel-phosphorus aloy, Corrosion, 56(2000), No. 3, p. 283.
[12] Z. Zhang, W.H. Leng, J.F. Li, J.Q. Zhang, J.M. Wang, and C.N. Cao, Cooperation behavior of iron and phosphorus in electrodeposition of zinc-iron-phosphorus coating, Mater. Chem. Phys., 77(2003), No. 2, p. 497.
[13] M.M. Younan and T. Oki, Electrodeposition of Zn-Ni-Fe alloy in acidic chloride bath with separated anodes, J. Appl. Electrochem., 26(1996), No. 5, p. 537.
[14] M. M. Abou-Krisha, F. H. Assaf and S. A. El-Naby, Electrodeposition behavior of zinc-nickel-iron alloys from sulfate bath, J. Coat. Technol. Res., 6(2009), p. 391.
[15] M.M. Abou-Krisha, F.H. Assaf, and S.A. El-Naby, Electrodeposition and characterization of zinc-nickel-iron alloy from sulfate bath: Influence of platingbath temperature, J. Solid State Electrochem., 13(2009), No. 6, p. 879.
[16] M.M. Younan, Surface microstructure and corrosion resistance of electrodeposited ternary Zn-Ni-Co alloy, J. Appl. Electrochem., 30(2000), No. 1, p. 55.
[17] M.M. Abou-Krisha, H.M. Rageh, and EA. Matter, Electrochemical studies on the electrodeposited Zn-Ni-Co ternary alloy in different media, Surf. Coat. Technol, 202(2008), No. 15, p. 3739.
[18] N. Eliaz, K. Venkatakrishna, and A.C. Hegde, Hegde, Electroplating and characterization of Zn-Ni, Zn-Co and Zn-Ni-Co alloys, Surf. Coat. Technol, 205(2010), No. 7, p. 1969.
[19] J. Vijayakumar, S. Mohan, S.A. Kumar, S.R. Suseendiran, and S. Pavithra, Electrodeposition of Ni-Co-Sn alloy from choline chloride-based deep eutectic solvent and characterization as cathode for hydrogen evolution in alkaline solution, Int. J. Hydrogen Energy, 38(2013), No. 25, p. 10208.
[20] ZY. Zhou and T.J. O’Keefe, Modification of anomalous deposition of Zn-Ni alloy by using tin additions, Surf Coat. Technol, 96(1997), No. 2–3, p. 191.
[21] S. Fashu and R. Khan, Electrodeposition of ternary Zn-Ni-Sn alloys from an ionic liquid based on choline chloride and their characterisation, Trans. IMF, 94(2016), No. 5, p. 237.
[22] R. Solmaz and B.D. Karahan, Effect of tin additions on the corrosion behaviors of zinc-nickel coatings, [in] 19th International Metallurgy and Materials Congress Proceeding, Istanbul, 2018, p. 1224.
[23] C.M.K. PraveenKumar, T.V. Venkatesha, K. Vathsala, and K.O. Nayana, Electrodeposition and corrosion behavior of Zn-Ni and Zn-Ni-Fe2O3 coatings, J. Coat. Technol. Res. 9(2012), No. 1, p. 71.
[24] S. Fashu, C. Gu, J.L. Zhang, M.L. Huang, X. Wang and J. Tu, Effect of EDTA and NH4Cl additives on electrodeposition of Zn-Ni films from choline chloride-based ionic liquid, Trans. Nonferrous Met. Soc. China, 25(2015), No. 6, p. 2054.
[25] CF. Han, Q. Liu, and DG. Ivey, Kinetics of Sn electrode-position from Sn(II)-citrate solutions, Electrochim. Acta, 53(2008), No. 28, p. 8332.
[26] S.T. Bahade, A.S. Lanje, and S.J. Sharma, Synthesis of SnO2 thin film by sol-gel spin coating technique for optical and ethanol gas sensing application, Int. J. Sci. Eng. Res., 3(2017), No. 7, p. 567.
[27] K. Saoud, R. Alsoubaihi, N. Bensalah, T. Bora, M. Bertino, and J. Dutta, Synthesis of supported silver nano-spheres on zinc oxide nanorods for visible light photocatalytic applications, Mater. Res. Bull, 63(2015), p. 134.
[28] A.N. Kadam, D.P. Bhopate, V.V. Kondalkar, S.M. Majhi, CD. Bathula, A.V. Tran, and S.W. Lee, Facile synthesis of Ag-ZnO core-shell nanostructures with enhanced pho tocatalytic activity, J. Ind. Eng. Chem., 61(2018), p. 78.
[29] K. Handore, S. Bhavsar, A. Horne, P. Chhattise, K. Mohite, J. Ambekar, N. Pande, and V. Chabukswar, Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes, J. Macromol. Sci. Part A, 51(2014), No. 12, p. 941.
[30] F. Wolfart, D.P. Dubal, M. Vidotti, and P. G′omez-Romero, Hybrid core-shell nanostructured electrodes made of polypyrrole nanotubes coated with Ni(OH)2 nanoflakes for high energy-density supercapacitors, RSC Adv. 6(2016), No. 18, p. 15062.
[31] A.S. Adekunle, J.A.O. Oyekunle, O.S. Oluwafemi, A.O. Joshua, A.O. Makinde, A.O. Ogunfowokan, MA. Eleruja, and EE. Ebenso, Comparative catalytic properties of Ni(OH)2 and NiO nanoparticles towards the degradation of nitrite (NO2-) and nitric oxide (NO), Int. J. Electrochem. Sci., 9(2014), p. 3008.
[32] N.V. Krstajić, V.D. Jović, Lj. Gajić-Krstajić, B.M. Jović, AL. Antozzi, and G.N. Martelli, Electrodeposition of Ni-Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution, Int. J. Hydrogen Energy, 33(2008), No. 14, p. 3676.
[33] S. Costovici A.C. Manea, T. Visan, and L. Anicai, Investigation of Ni-Mo and Co-Mo alloys electrodeposition involving choline chloride based ionic liquids, Electrochim. Acta, 207(2016), p. 97.
[34] J. García-Antón, R.M. Fernández-Domene, R. Sánchez-To-var, C. Escrivà-Cerdán, R. Leiva-García, V. García, and A. Urtiaga, Improvement of the electrochemical behaviour of Zn-electroplated steel using regenerated Cr (III) passivation baths, Chem. Eng Sci, 111(2014), p. 402.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solmaz, R., Karahan, B.D. Characterization and corrosion studies of ternary Zn−Ni−Sn alloys. Int J Miner Metall Mater 27, 74–82 (2020). https://doi.org/10.1007/s12613-019-1888-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1888-4