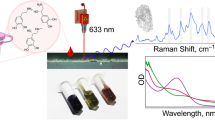
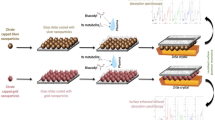
Abstract
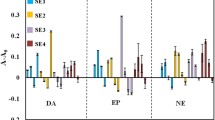
Surface-enhanced Raman spectroscopy (SERS) is a powerful analytical technique capable of increasing the Raman signal of an analyte using specific nanostructures. The close contact between those nanostructures, usually a suspension of nanoparticles, and the molecule of interest produces an important exaltation of the intensity of the Raman signal. Even if the exaltation leads to an improvement of Raman spectroscopy sensitivity, the complexity of the SERS signal and the numbers of parameters to be controlled allow the use of SERS for detection rather than quantification. The aim of this study was to develop a robust discriminative and quantitative analysis in accordance with pharmaceutical standards. In this present work, we develop a discriminative and quantitative analysis based on the previous optimized parameters obtained by the design of experiments fixed for norepinephrine (NOR) and extended to epinephrine (EPI) which are two neurotransmitters with very similar structures. Studying the short evolution of the Raman signal intensity over time coupled with chemometric tools allowed the identification of outliers and their removal from the data set. The discriminant analysis showed an excellent separation of EPI and NOR. The comparative analysis of the data showed the superiority of the multivariate analysis after logarithmic transformation. The quantitative analysis allowed the development of robust quantification models from several gold nanoparticle batches with limits of quantification of 32 µg/mL for NOR and below 20 µg/mL for EPI even though no Raman signal is observable for such concentrations. This study improves SERS analysis over ultrasensitive detection for discrimination and quantification using a handheld Raman spectrometer.
Graphical abstract











Similar content being viewed by others
References
Michelet A, Boiret M, Lemhachheche F, Malec L, Tfayli A, Ziemons E. Utilisation de la spectrométrie Raman dans le domaine pharmaceutique. STP Pharma Prat. 2013;23(2):97–117.
Lê L, Berge M, Tfayli A, Prognon P, Caudron E. Discriminative and quantitative analysis of antineoplastic Taxane drugs using a handheld Raman spectrometer. BioMed Res Int. 2018;2018:1–7.
Shende C, Smith W, Brouillette C, Farquharson S. Drug stability analysis by Raman spectroscopy. Pharmaceutics déc. 2014;6(4):651–62.
Parachalil DR, McIntyre J, Byrne HJ. Potential of Raman spectroscopy for the analysis of plasma/serum in the liquid state: recent advances. Anal Bioanal Chem. 2020;412(9):1993–2007.
Lê L, Berge M, Tfayli A, BailletGuffroy A, Prognon P, Dowek A, et al. Quantification of gemcitabine intravenous drugs by direct measurement in chemotherapy plastic bags using a handheld Raman spectrometer. Talanta. 2019;196:376–80.
Mansouri MA, Sacré P-Y, Coïc L, De Bleye C, Dumont E, Bouklouze A, et al. Quantitation of active pharmaceutical ingredient through the packaging using Raman handheld spectrophotometers: a comparison study. Talanta. 2020;207:120306.
Lê LMM, Berge M, Tfayli A, Zhou J, Prognon P, Baillet-Guffroy A, et al. Rapid discrimination and quantification analysis of five antineoplastic drugs in aqueous solutions using Raman spectroscopy. Eur J Pharm Sci. 2018;111:158–66.
Sharma B, Frontiera RR, Henry A-I, Ringe E, Van Duyne RP. SERS: materials, applications, and the future. Mater Today. 2012;15(1–2):16–25.
Kneipp K, Kneipp H, Manoharan R, Itzkan I, Dasari RR, Feld MS. Near-infrared surface-enhanced Raman scattering can detect single molecules and observe ‘hot’ vibrational transitions. J Raman Spectrosc. 1998;29(8):743–7.
Cailletaud J, De Bleye C, Dumont E, Sacré P-Y, Netchacovitch L, Gut Y, et al. Critical review of surface-enhanced Raman spectroscopy applications in the pharmaceutical field. J Pharm Biomed Anal. 2018;147:458–72.
Goodacre R, Graham D, Faulds K. Recent developments in quantitative SERS: Moving towards absolute quantification. TrAC Trends Anal Chem. 2018;102:359–68.
Muhamadali H, Watt A, Xu Y, Chisanga M, Subaihi A, Jones C, et al. Rapid Detection and Quantification of Novel Psychoactive Substances (NPS) Using Raman Spectroscopy and Surface-Enhanced Raman Scattering. Front Chem [Internet]. 2019 [cité 29 mai 2021];7. Disponible sur: https://www.frontiersin.org/articles/https://doi.org/10.3389/fchem.2019.00412/full
Junior BRA, Soares FLF, Ardila JA, Durango LGC, Forim MR, Carneiro RL. Determination of B-complex vitamins in pharmaceutical formulations by surface-enhanced Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2018;188:589–95.
Markina NE, Goryacheva IY, Markin AV. Sample pretreatment and SERS-based detection of ceftriaxone in urine. Anal Bioanal Chem. 2018;410(8):2221–7.
Alharbi O, Xu Y, Goodacre R. Simultaneous multiplexed quantification of caffeine and its major metabolites theobromine and paraxanthine using surface-enhanced Raman scattering. Anal Bioanal Chem. 2015;407(27):8253–61.
De Bleye C, Dumont E, Rozet E, Sacré P-Y, Chavez P-F, Netchacovitch L, et al. Determination of 4-aminophenol in a pharmaceutical formulation using surface enhanced Raman scattering: From development to method validation. Talanta. 2013;15(116):899–905.
Alharbi O, Xu Y, Goodacre R. Detection and quantification of the opioid tramadol in urine using surface enhanced Raman scattering. Analyst. 2015;140(17):5965–70.
Dowek A, Lê LMM, Rohmer T, Legrand F-X, Remita H, Lampre I, et al. A mathematical approach to deal with nanoparticle polydispersity in surface enhanced Raman spectroscopy to quantify antineoplastic agents. Talanta. 2020;217:121040.
Fan M, Andrade GF, Brolo AG. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal Chim Acta. 2011;693(1–2):7–25.
Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.
Lee PC, Meisel D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem. 1982;86(17):3391–5.
Shi L, Buhler E, Boué F, Carn F. How does the size of gold nanoparticles depend on citrate to gold ratio in Turkevich synthesis? Final answer to a debated question. J Colloid Interface Sci. 2017;492:191–8.
Li W, Zhao X, Yi Z, Glushenkov AM, Kong L. Plasmonic substrates for surface enhanced Raman scattering. Anal Chim Acta. 2017;984:19–41.
Tian F, Bonnier F, Casey A, Shanahan AE, Byrne HJ. Surface enhanced Raman scattering with gold nanoparticles: effect of particle shape. Anal Methods. 2014;6(22):9116–23.
Fisk H, Westley C, Turner NJ, Goodacre R. Achieving optimal SERS through enhanced experimental design: achieving SERS by enhanced experimental design. J Raman Spectrosc. 2016;47(1):59–66.
Cailletaud J, De Bleye C, Dumont E, Sacré P-Y, Gut Y, Leblanc N, et al. Detection of low dose of piroxicam polymorph in pharmaceutical tablets by surface-enhanced Raman chemical imaging (SER-CI) and multivariate analysis. Int J Pharm. 2019;574:118913.
Eliasson C, Lorén A, Murty KVGK, Josefson M, Käll M, Abrahamsson J, et al. Multivariate evaluation of doxorubicin surface-enhanced Raman spectra. Spectrochim Acta A Mol Biomol Spectrosc. 2001;57(9):1907–15.
Deng B, Luo X, Zhang M, Ye L, Chen Y. Quantitative detection of acyclovir by surface enhanced Raman spectroscopy using a portable Raman spectrometer coupled with multivariate data analysis. Colloids Surf B Biointerfaces. 2019;173:286–94.
Weng S, Yu S, Dong R, Zhao J, Liang D. Detection of Pirimiphos-Methyl in Wheat Using Surface-Enhanced Raman Spectroscopy and Chemometric Methods. Mol Basel Switz. 2019;24(9):1691.
Dijkstra RJ, Scheenen WJJM, Dam N, Roubos EW, ter Meulen JJ. Monitoring neurotransmitter release using surface-enhanced Raman spectroscopy. J Neurosci Methods. 2007;159(1):43–50.
Moody AS, Sharma B. Multi-metal, Multi-wavelength Surface-Enhanced Raman Spectroscopy Detection of Neurotransmitters. ACS Chem Neurosci. 2018;9(6):1380–7.
Shi C-X, Chen Z-P, Chen Y, Liu Q, Yu R-Q. Quantification of dopamine in biological samples by surface-enhanced Raman spectroscopy: Comparison of different calibration models. Chemom Intell Lab Syst. 2017;169:87–93.
Hubert Ph, Nguyen-Huu J-J, Boulanger B, Chapuzet E, Chiap P, Cohen N, et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal – Part II. J Pharm Biomed Anal. 2007;45(1):70–81.
Eilers, Paul HC, Hans FM Boelens. Baseline correction with asymmetric least squares smoothing. Leiden University Medical Centre Report 1.1 (2005):5.
Olivieri AC. Practical guidelines for reporting results in single-and multi-component analytical calibration: a tutorial. Anal Chim Acta. 2015;868:10–22.
Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58(2):109–30.
Barker M, Rayens W. Partial least squares for discrimination. J Chemom. 2003;17(3):166–73.
Lee NS, Hsieh YZ, Paisley RF, Morris MD. Surface-enhanced Raman spectroscopy of the catecholamine neurotransmitters and related compounds. Anal Chem. 1988;60(5):442–6.
Moody AS, Baghernejad PC, Webb KR, Sharma B. Surface enhanced spatially offset Raman spectroscopy detection of neurochemicals through the skull. Anal Chem. 2017;89(11):5688–92.
Munro CH, Smith WE, Garner M, Clarkson J, White PC. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering. Langmuir. 1995;11(10):3712–20.
Park J-W, Shumaker-Parry JS. Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles. J Am Chem Soc. 2014;136(5):1907–21.
Moody AS, Payne TD, Barth BA, Sharma B. Surface-enhanced spatially-offset Raman spectroscopy (SESORS) for detection of neurochemicals through the skull at physiologically relevant concentrations. Analyst. 2020;145(5):1885–93.
Moody AS, Sharma B. Multi-metal, multi-wavelength surface-enhanced Raman spectroscopy detection of neurotransmitters. ACS Chem Neurosci. 2018;9(6):1380–7.
Vander Ende E, Bourgeois MR, Henry A-I, Chávez JL, Krabacher R, Schatz GC, et al. Physicochemical Trapping of Neurotransmitters in Polymer-Mediated Gold Nanoparticle Aggregates for Surface-Enhanced Raman Spectroscopy. Anal Chem. 2019;91(15):9554–62.
Acknowledgements
The authors would like to acknowledge financial support for a Nan’eau microscope from Ecole Polytechnique, IDEX Paris-Saclay, Equipex Morphoscope 2, and DGA. They also thank the “Centre Interdisciplinaire de Microscopie électronique de l’X” (CIMEX). Finally, they are grateful to the Fondation ARC (French foundation for cancer research) for the financial support to Marion Berge.
Funding
This work has been supported by the Fondation ARC pour la recherche.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dowek, A., Berge, M., Prognon, P. et al. Discriminative and quantitative analysis of norepinephrine and epinephrine by surface-enhanced Raman spectroscopy with gold nanoparticle suspensions. Anal Bioanal Chem 414, 1163–1176 (2022). https://doi.org/10.1007/s00216-021-03743-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03743-4