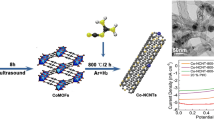
Abstract
The low cost and highly efficient construction of electrocatalysts has attracted significant attention owing to the use of clean and sustainable energy technologies. In this work, cobalt nanoparticle decorated N-doped carbons (Co@NC) are synthesized by the pyrolysis of a cobalt covalent organic framework under an inert atmosphere. The Co@NC demonstrates improved electrocatalytic capabilities compared to N-doped carbon without the addition of Co nanoparticles, indicating the important role of cobalt. The well-dispersed active sites (Co-Nx) and the synergistic effect between the carbon matrix and Co nanoparticles greatly enhance the electrocatalytic activity for the oxygen reduction reaction. In addition, the Co content has a significant effect on the catalytic activity. The resulting Co@NC-0.86 exhibits a superb electrocatalytic activity for the oxygen reduction reaction in an alkaline electrolyte in terms of the onset potential (0.90 V), half-wave potential (0.80 V) and the limiting current density (4.84 mA·cm−2), and a high selectivity, as well as a strong methanol tolerance and superior durability, these results are comparable to those of the Pt/C catalyst. Furthermore, the superior bifunctional activity of Co@NC-0.86 was also confirmed in a home-built Zn-air battery, signifying the possibility for application in electrode materials and in current energy conversion and storage devices.

Similar content being viewed by others
References
Zhang H X, Liang J Y, Xia B W, Li Y, Du S F. Ionic liquid modified Pt/C electrocatalysts for cathode application in proton exchange membrane fuel cells. Frontiers of Chemical Science and Engineering, 2019, 13(4): 695–701
Peng X F, Wang Z H, Wang Z, Pan Y X. Multivalent manganese oxides with high electrocatalytic activity for oxygen reduction reaction. Frontiers of Chemical Science and Engineering, 2018, 12 (4): 790–797
Hao R, Ren J T, Lv X W, Li W, Liu Y P, Yuan Z Y. N-Doped porous carbon hollow microspheres encapsulated with iron-based nano-composites as advanced bifunctional catalysts for rechargeable Zn-air battery. Journal of Energy Chemistry, 2020, 49: 14–21
Yin S H, Yang J, Han Y, Li G, Wan L Y, Chen Y H, Chen C, Qu X M, Jiang Y X, Sun S G. Construction of highly active metal-containing nanoparticles and FeCo-N4 composite sites for the acidic oxygen reduction reaction. Angewandte Chemie International Edition, 2020, 59(49): 21976–21979
Ren J T, Yuan Z Y. A universal route to N-coordinated metals anchored on porous carbon nanosheets for highly efficient oxygen electrochemistry. Journal of Materials Chemistry A, 2019, 7(22): 13591–13601
Wu L M, Ni B X, Chen R, Sun P C, Chen T H. A general approach for hierarchically porous metal/N/C nanosphere electrocatalysts: nano-confined pyrolysis of in situ-formed amorphous metal-ligand complexes. Journal of Materials Chemistry A, 2020, 8(40): 21026–21035
Medard C, Lefevre M, Dodelet J, Jaouen F, Lindbergh G. Oxygen reduction by Fe-based catalysts in PEM fuel cell conditions: activity and selectivity of the catalysts obtained with two Fe precursors and various carbon supports. Electrochimica Acta, 2006, 51(16): 3202–3213
Wang H G, Weng C C, Yuan Z Y. Insights into efficient transition metal-nitrogen/carbon oxygen reduction electrocatalysts. Journal of Energy Chemistry, 2021, 56: 470–485
Zhao L M, Liu H M, Du Y, Liang X, Wang W J, Zhao H, Li W Z. An ionic liquid as a green solvent for high potency synthesis of 2D covalent organic frameworks. New Journal of Chemistry, 2020, 44 (36): 15410–15414
Liang X, Liu H M, Du Y, Li W Z, Wang M, Ge B, Zhao L M. Terbium functionalized covalent organic framework for selective and sensitive detection of LVX based on fluorescence enhancement. Colloids and Surfaces A, 2020, 606: 125429
Sharma R K, Yadav P, Yadav M, Gupta R, Rana P, Srivastava A, Zbořil R, Varma R S, Antonietti M, Gawande M B. Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications. Materials Horizons, 2020, 7(2): 411–454
Rodríguez-San-Miguel D, Montoro C, Zamora F. Covalent organic framework nanosheets: preparation, properties and applications. Chemical Society Reviews, 2020, 49(8): 2291–2302
Li H, Chen F Q, Guan X Y, Li J L, Li C Y, Tang B, Valtchev V, Yan Y S, Qiu S L, Fang Q R. Three-dimensional triptycene-based covalent organic frameworks with ceq or acs topology. Journal of the American Chemical Society, 2021, 143(1): 2654–2659
Cui X, Lei S, Wang A C, Gao L K, Zhang Q, Yang Y K, Lin Z Q. Emerging covalent organic frameworks tailored materials for electrocatalysis. Nano Energy, 2020, 70: 104525
Yusran Y, Fang Q R, Valtchev V. Electroactive covalent organic frameworks: design, synthesis, and applications. Advanced Materials, 2020, 32(44): 2002038
Wang D, Qiu T, Guo W, Liang Z, Tabassum H, Xia D, Zou R. Covalent organic framework-based materials for energy applications. Energy & Environmental Science, 2021, 14(2): 688–728
Wang J, Wang J R, Qi S Y, Zhao M W. Stable multifunctional single-atom catalysts resulting from the synergistic effect of anchored transition-metal atoms and host covalent-organic frameworks. Journal of Physical Chemistry C, 2020, 124(32): 17675–17683
Wei S J, Wang Y, Chen W X, Li Z, Cheong W C, Zhang Q H, Gong Y, Gu L, Chen C, Wang D S, et al. Atomically dispersed Fe atoms anchored on COF-derived N-doped carbon nanospheres as efficient multi-functional catalysts. Chemical Science (Cambridge), 2020, 11 (3): 786–790
Roy S, Mari S, Sai M K, Sarma S C, Sarkar S, Peter S C. Highly efficient bifunctional oxygen reduction/evolution activity of a non-precious nanocomposite derived from a tetrazine-COF. Nanoscale, 2020, 12(44): 22718–22734
Zhu Y Z, Peng W C, Li Y, Zhang G L, Zhang F B, Fan X B. Modulating the electronic structure of single-atom catalysts on 2D nanomaterials for enhanced electrocatalytic performance. Small Methods, 2019, 3(9): 1800438
Kandambeth S, Mallick A, Lukose B, Mane M V, Heine T, Banerjee R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. Journal of the American Chemical Society, 2012, 134(48): 19524–19527
Zhao H, Hu Z P, Zhu Y P, Ge L, Yuan Z Y. P-doped mesoporous carbons for high-efficiency electrocatalytic oxygen reduction. Chinese Journal of Catalysis, 2019, 40(9): 1366–1374
Zhao H, Weng C C, Ren J T, Ge L, Liu Y P, Yuan Z Y. Phosphonate-derived nitrogen-doped cobalt phosphate/carbon nanotube hybrids as highly active oxygen reduction reaction electrocatalysts. Chinese Journal of Catalysis, 2020, 41(2): 259–267
Yang Z, Zhao C, Qu Y, Zhou H, Zhou F, Wang J, Wu Y, Li Y. Trifunctional self-supporting cobalt-embedded carbon nanotube films for ORR, OER, and HER triggered by solid diffusion from bulk metal. Advanced Materials, 2019, 31(12): 1808043
Lv X W, Liu Y, Wang Y S, Liu X L, Yuan Z Y. Encapsulating vanadium nitride nanodots into N,S-codoped graphitized carbon for synergistic electrocatalytic nitrogen reduction and aqueous Zn-N2 battery. Applied Catalysis B: Environmental, 2021, 280: 119434
Weng C C, Ren J T, Hu Z P, Yuan Z Y. Nitrogen-doped defect-rich graphitic carbon nanorings with CoOx nanoparticles as highly efficient electrocatalyst for oxygen electrochemistry. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15811–15821
Ouyang T, Ye Y Q, Wu C Y, Xiao K, Liu Z Q. Heterostructures comprised of Co/β-Mo2C-encapsulated N-doped carbon nanotubes as bifunctional electrodes for water splitting. Angewandte Chemie International Edition, 2019, 58(15): 4923–4928
Aijaz A, Masa J, Rösler C, Xia W, Weide P, Botz A J R, Fischer R A, Schuhmann W, Muhler M. Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angewandte Chemie International Edition, 2016, 55(12): 4087–4091
Wang Q, Zhou Z Y, Lai Y J, You Y, Liu J G, Wu X L, Terefe E, Chen C, Song L, Rauf M, et al. Phenylenediaminebased FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. Journal of the American Chemical Society, 2014, 136(31): 10882–10885
Lefèvre M, Proietti E, Jaouen F, Dodelet J P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science, 2009, 324(5923): 71–74
Lai L, Potts J R, Zhan D, Wang L, Poh C K, Tang C, Gong H, Shen Z, Lin J, Ruoff R S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy & Environmental Science, 2012, 5(7): 7936–7942
Guo D, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science, 2016, 351(6271): 361–365
Wu G, More K L, Johnston C M, Zelenay P. High performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science, 2011, 332(6028): 443–447
Cheon J Y, Kim J H, Kim J H, Goddeti K C, Park J Y, Joo S H. Intrinsic relationship between enhanced oxygen reduction reaction activity and nanoscale work function of doped carbons. Journal of the American Chemical Society, 2014, 136(25): 8875–8878
Zhang Y T, Wang P, Yang J, Lu S S, Li K K, Liu G Y, Duan Y F, Qiu J S. Decorating ZIF-67-derived cobalt-nitrogen doped carbon nanocapsules on 3D carbon frameworks for efficient oxygen reduction and oxygen evolution. Carbon, 2021, 177: 344–356
Sa Y J, Park S O, Jung G Y, Shin T J, Jeong H Y, Kwak S K, Joo S H. Heterogeneous Co-N/C electrocatalysts with controlled cobalt site densities for the hydrogen evolution reaction: structure-activity correlations and kinetic insights. ACS Catalysis, 2019, 9 (1): 83–97
Liu Y, Song C Y, Wang Y C, Cao W H, Lei Y P, Feng Q G, Chen Z, Liang S J, Xu L, Jiang L L. Rational designed Co@N-doped carbon catalyst for high-efficient H2S selective oxidation by regulating electronic structures. Chemical Engineering Journal, 2020, 401: 126038
Tan Y, Xu C, Chen G, Fang X, Zheng N, Xie Q. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction. Advanced Functional Materials, 2012, 22(21): 4584–4591
Liu S, Wang Z, Zhou S, Yu F, Yu M, Chiang C Y, Zhou W, Zhao J, Qiu J. Metal-organic-framework-derived hybrid carbon nanocages as a bifunctional electrocatalyst for oxygen reduction and evolution. Advanced Materials, 2017, 29(31): 1700874–1700883
Yang H B, Miao J W, Hung S F, Chen J Z, Tao H B, Wang X Z, Zhang L P, Chen R, Gao J J, Chen H M, et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: development of highly efficient metal-free bifunctional electrocatalyst. Science Advances, 2016, 2(4): e1501122
Masa J, Xia W, Muhler M, Schuhmann W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angewandte Chemie International Edition, 2015, 54(35): 10102–10120
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2019PB013), the Training Program of Innovation and Entrepreneurship for Undergraduates (Grant No. CXCY2021161), the Natural Science Foundation of Tianjin (Grant No. 19JCZDJC37700), and the National Natural Science Foundation of China (Grant No. 21875118).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
11705_2021_2104_MOESM1_ESM.pdf
Cobalt nanoparticle decorated N-doped carbons derived from a cobalt covalent organic framework for oxygen electrochemistry
Rights and permissions
About this article
Cite this article
Zhang, RQ., Ma, A., Liang, X. et al. Cobalt nanoparticle decorated N-doped carbons derived from a cobalt covalent organic framework for oxygen electrochemistry. Front. Chem. Sci. Eng. 15, 1550–1560 (2021). https://doi.org/10.1007/s11705-021-2104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-021-2104-4