Abstract
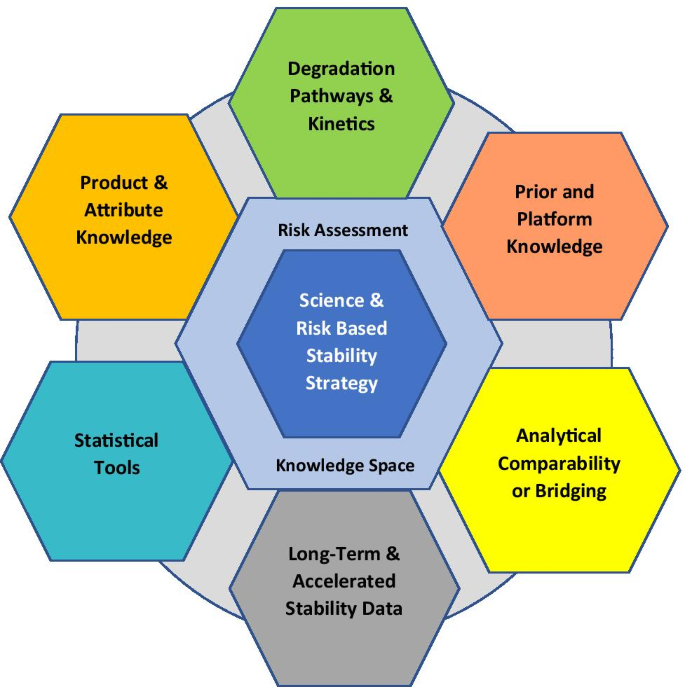
In consideration of the recent ICH Quality Discussion Group (QDG) recommended revision to the ICH series of stability guidelines, the IQ Consortium (International Consortium for Innovation and Quality in Pharmaceutical Development) Science- and Risk-based Stability Working Group conducted a comprehensive review of ICH Q1A, Q1B, Q1C, Q1D, Q1E, and Q5C to identify areas where the guidelines could be clarified, updated, and amended to reflect the potential knowledge gained from current risk-based predictive stability tools and to consider other science- and risk-based stability strategies in accordance with ICH Q8–12. The recommendations propose a holistic approach to stability understanding, utilizing historical data, prior knowledge, modeling, and a risk assessment process to expand the concept of what could be included (or would be acceptable) in the core stability data package, including type and amount of stability evidence, assignment of retest period and shelf-life for a new product, and assessment of the impact of post-approval changes.
Graphical abstract
Similar content being viewed by others
Change history
15 November 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00646-1
References
ICH Harmonised Tripartite Guideline. “Stability testing of new drug substances and products Q1A (R2).” Current Step 4: February (2003).
ICH Harmonised Tripartite Guideline. “Photostability testing of new drug substances and products Q1B.” Current Step 4: November (1996)
ICH Harmonised Tripartite Guideline. “Annex to the ICH harmonised tripartite guideline on stability testing for new drugs and products Q1C.” Current Step 4 November (1996)
ICH Harmonised Tripartite Guideline. “Bracketing and matrixing designs for stability testing of new drug substances and products Q1D.” Current Step 4: February (2002).
ICH Harmonised Tripartite Guideline. “Evaluation for stability data Q1E.” Current Step 4: February (2003).
ICH Harmonised Tripartite Guideline. “Stability data package for registration applications in climatic zones III and IV Q1F; Explanatory Note on the Withdrawal of ICH Q1F for the ICH Website”
ICH Harmonised Tripartite Guideline. “Quality of biotechnological products: stability testing of biotechnological/biological products Q5C.” Current Step 4: November (1995).
ICH Harmonised Tripartite Guideline. “Pharmaceutical development Q8(R2)” Current Step 4 August (2009).
ICH Harmonised Tripartite Guideline. “Quality risk management Q9.” Current Step 4 November (2005).
ICH Harmonised Tripartite Guideline. “Pharmaceutical quality system Q10.” Current Step 4 June (2008).
ICH Harmonised Tripartite Guideline. “Development and manufacture of drug substances (chemical entities and biotechnological/biological entities) Q11.” Current Step 4: May (2012).
ICH Harmonised Tripartite Guideline. “Technical and regulatory considerations for pharmaceutical product lifecycle management Q12.” Final version Adopted November (2019).
Waterman KC, Carella AJ, Gumkowski MJ, Lukulay P, MacDonald BC, Roy MC, Shamblin SL. Improved protocol and data analysis for accelerated shelf-life estimation of solid dosage forms, Pharm Res; April 2007; 24 (4): 780–790.
Clancy D, Hodnett NS, Orr R, Owen M, Peterson J. Kinetic model development for accelerated stability studies. AAPS PharmSciTech. 2017;18(4):1158–76.
Qiu F, Scrivens G. Accelerated predictive stability (APS): fundamentals and pharmaceutical industry practices, 1st Edition, Academic Press; May 30, 2018, Cambridge, MA, USA
Wu Y, McMahon M, Regler B. Applications of accelerated stability models in product development. (November 4, 2018) PharmSci360 short course. https://www.conferenceharvester.com/Uploads/Documents/HarvesterLink-5946-748(41).pdf
Colgan ST, Timpano RJ, Roberts M, Weaver R, Ryan K, Fields K. SRA opportunities for lean stability strategies. J Pharm Innov. 2014;9:259–71.
Colgan S, Hoffer J, Timpano R, Vukovinsky K, Waterman K, Norris K. Lean stability. AAPS News Magazine, September (2015).
Clénet D, Imbert F, Probeck P, Rahman N, Ausar SF. Advanced kinetic analysis as a tool for formulation development and prediction of vaccine stability. J Pharm Sci. 2014;103:3055–64.
Roduit B, Hartmann M, Folly P, Sarbach A, Baltensperger R. Prediction of thermal stability of materials by modified kinetic and model selection approaches based on limited amount of experimental points. Thermochim Acta. 2014;579:31–9.
Clénet F. Accurate prediction of vaccine stability under real storage conditions and during temperature excursions. Eur J Pharm Biopharm. 2018;125:76–84.
Roduit B, Luyet CA, Hartmann M, Folly P, Sarbach A, Dejeaifve A, et al. Continuous monitoring of shelf lives of materials by application of data loggers with implemented kinetic parameters. M Molecules. 2019;24(12):2217.
Roque C, Ausar SF, Raham N, Clénet D, Stability modeling in QbD: accelerating formulation development and predicting shelf life of products, PDA book chapter dedicated on Quality by Design, ISBN number: 978–1–945584–22–0, 2021.
Williams H, Qiu F, Wu Y, Hahn D, McMahon M, Orr R, et al. Risk-based predictive stability–an industry perspective. Pharm Tech. 2017;41(3):52–7.
Campa C, Khan MA. Quality by design— an indispensable approach to accelerate biopharmaceutical product development (March, 2021): https://www.pda.org/bookstore/product-detail/6054-quality-by-design?utm_source=caboodle&utm_medium=email&utm_campaign=publications_qbd&utm_content=3.11.21
2019 Project Report: monitoring the adequacy of implementation and adherence to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (November 1, 2019): ICHImplementationReportOutline_v1.4_2019_1101.pdf
EMA PRIME (PRIority MEdicines): Launch of PRIME – paving the way for promising medicines for patients; 4 March 2016 EMA/89921/2016
Guideline on the scientific application and the practical arrangements necessary to implement Commission Regulation (EC) No 507/2006 on the conditional marketing authorisation for medicinal products for human use falling within the scope of Regulation (EC) No 726/2004; 25 February 2016 EMA/CHMP/509951/2006, Rev.1
FDA Breakthrough Therapy Designation: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy
FDA Guideline on Emergency Use Authorization of Medical Products and Related Authorities; January 2017, Procedural OMB Control No. 0910–0595, Expiration Date 08/31/2022
FDA Project Orbis: https://www.fda.gov/about-fda/oncology-center-excellence/project-orbis
Access Consortium: Terms of Reference: Australia, Canada, Singapore, Switzerland and United Kingdom Consortium (Access Consortium) (tga.gov.au)
Acknowledgements
The authors would like to thank the following colleagues for the thoughtful review comments they provided on this paper: Jin Wang, Chait Wannere, Barbara Rellahan, Beth Kendsersky, Kevin Ryan, Ron Ogilvie, Clarice Hutchens, Mark D. Argentine, Michael R. De Felippis, George Daniel Willingmyre, Siqing Song, Kelly Norton, Cheryl Pape, Paul Hermes, Carolyn Gordon, Anette Skoog, Richard Bradley, Angelica Wickström, Simon Lee, Timothy Watson, and Margaret Faul.
The authors wish to dedicate this article to Helen Williams, who passed away in August 2021 after a long illness. Helen was a founding member of the IQ Working Group for Risk Based Predictive Stability and played a fundamental role in the success and growth of the group, building connections and authoring several publications. As a leader and advocate for the emerging field of predictive stability, Helen championed the acceleration of pharmaceutical development and brought energy and determination to the task of reinventing established practice. Helen was a wonderful friend and colleague, and will be profoundly missed by the pharmaceutical stability community.
Funding
Not applicable; authors contributed case studies based on existing company knowledge and experience.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this manuscript and the detailed recommendations listed in the supplementary material. Chi-wan Chen authored the “Introduction” section. Alexander Abbott, Andrew Lennard, Lori McCaig, Jenny Carhart, Robert Timpano, Fenghe Qiu, Dennis Stephens, Hanlin Li, Megan McMahon, and Chi-wan Chen authored the “Recommendations” section. Lori McCaig authored the “Discussion” section. Megan McMahon authored the “Conclusion” section. All authors reviewed, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add the graphical abstract.
This paper was developed with the support of the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ, www.iqconsortium.org). IQ is a not-for-profit organization of pharmaceutical and biotechnology companies with a mission of advancing science and technology to augment the capability of member companies to develop transformational solutions that benefit patients, regulators, and the broader research and development community.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McMahon, M.E., Abbott, A., Babayan, Y. et al. Considerations for Updates to ICH Q1 and Q5C Stability Guidelines: Embracing Current Technology and Risk Assessment Strategies. AAPS J 23, 107 (2021). https://doi.org/10.1208/s12248-021-00641-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00641-6