Abstract
In this study, carotenoprotein from Pacific white shrimp (Litopenaeus vannamei) processing waste was extracted with the aid of alcalase (CP-A) and pepsin (CP-P) at various levels (0–4 units/100 g waste) for different times (0–240 min). Recovery of carotenoids and protein increased with 3 unit/g of enzyme and hydrolysis times until 180 min (p < 0.05). The extracted carotenoprotein by pepsin and alcalase consisted of 72.11–75.32% protein and carotenoids content was in the range of 330–530 µg/g samples. The phenylalanine, lysine, methionine, and valine as essential amino acids were higher at CP-A and CP-P. The dominant non-essential amino acids in carotenoproteins were aspartic acid, glutamic acid, glycine, and alanine. It was rich in mono and polyunsaturated fatty acids. The CP-A showed higher content of docosahexaenoic acid and eicosapentaenoic acid (8.52 and 6.49%) than CP-P (5.55 and 5.49%). The saturated fatty acids were reduced after enzymatic hydrolysis and contents were higher in carotenoproteins. The extracted samples showed a significant amount of mineral contents. Sodium, phosphorus, magnesium, and potassium contents were found higher in CP-A. The lead and copper were reduced as a result of hydrolysis. Therefore, carotenoprotein from processing residue of pacific white shrimp could be used as the value-added nutritious enriching food or feed powder.
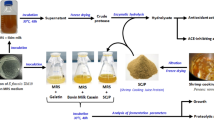
Graphic Abstract




Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CPs:
-
Carotenoproteins
- CP-A:
-
Carotenoprotein Recovered by Alcalase
- CP-P:
-
Carotenoprotein Recovered by Pepsin
- SW:
-
Shrimp Waste
- SFA:
-
Saturated Fatty Acids
- MUFA:
-
Mono Unsaturated Fatty Acids
- PUFA:
-
Poly Unsaturated Fatty Acids
- BSA:
-
Bovine Serum Albumin
- BF3:
-
Boron triFluoride
- FAMEs:
-
Fatty Acid Methyl Esters
References
Morales-Medina, R., et al.: Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem. 194, 1208–1216 (2016)
Sila, A., Nasri, M., Bougatef, A.: Isolation and characterisation of carotenoproteins from deep-water pink shrimp processing waste. Int. J. Biol. Macromol. 51(5), 953–959 (2012)
Sila, A., et al.: Ability of natural astaxanthin from shrimp by-products to attenuate liver oxidative stress in diabetic rats. Pharmacol. Rep. 67(2), 310–316 (2015)
Gómez-Estaca, J., et al.: Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 216, 37–44 (2017)
Sowmya, R., Rathinaraj, K., Sachindra, N.M.: An autolytic process for recovery of antioxidant activity rich carotenoprotein from shrimp heads. Mar. Biotechnol. 13(5), 918–927 (2011)
Sachindra, N.M., Bhaskar, N., Mahendrakar, N.S.: Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 26(10), 1092–1098 (2006)
FAO: The state of world fisheries and aquaculture. In: Sustainability in Action. FAO, Rome (2020)
Takeungwongtrakul, S., Benjakul, S.: Oxidative stability of lipids from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) as affected by essential oils incorporation. Eur. J. Lipid Sci. Technol. 116(8), 987–995 (2014)
Nasri, R., et al.: Digestive alkaline proteinases from Serranus scriba viscera: characteristics, application in the extraction of carotenoproteins from shrimp waste, and evaluation in laundry commercial detergents. Biocatal. Agric. Biotechnol. 4(3), 355–361 (2015)
Mechri, S., et al.: A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environ. Sci. Pollut. Res. 27(13), 15842–15855 (2020)
Sowmya, R., Sachindra, N.M.: Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chem. 134(1), 308–314 (2012)
Senphan, T., Benjakul, S., Kishimura, H.: Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci. 5, 54–63 (2014)
Pattanaik, S.S., et al.: Characterization of carotenoprotein from different shrimp shell waste for possible use as supplementary nutritive feed ingredient in animal diets. Aquaculture 515, 734594 (2020)
Chakrabarti, R.: Carotenoprotein from tropical brown shrimp shell waste by enzymatic process. Food Biotechnol. 16(1), 81–90 (2002)
Armenta, R.E., Guerrero-Legarreta, I.: Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 112(2), 310–315 (2009)
Kannan, A., et al.: Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation. J. Sci. Food Agric. 91(10), 1920–1924 (2011)
Gildberg, A., Stenberg, E.: A new process for advanced utilisation of shrimp waste. Process Biochem. 36(8), 809–812 (2001)
Poonsin, T., et al.: Albacore tuna spleen trypsin: potential application as laundry detergent additive and in carotenoprotein extraction from Pacific white shrimp shells. Biocatal. Agric. Biotechnol. 17, 638–646 (2019)
Poonsin, T., et al.: Carotenoprotein from Pacific white shrimp (Litopenaeus vannamei) shells extracted using trypsin from albacore tuna (Thunnus alalunga) spleen: antioxidant activity and its potential in model systems. J. Food Biochem. 42(2), e12462 (2018)
Gornall, A.G., Bardawill, C.J., David, M.M.: Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177(2), 751–766 (1949)
AOAC: Official Methods of Analysis. AOAC, Gaithersburg (2002)
Metcalfe, L.D., Schmitz, A.A.: The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 33(3), 363–364 (1961)
Sánchez-Camargo, A.P., et al.: Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 102(1), 87–93 (2011)
Gómez-Estaca, J., Montero, P., Gómez-Guillén, M.C.: Chemical characterization of wash water biomass from shrimp surimi processing and its application to develop functional edible films. J. Food Sci. Technol. 55(10), 3881–3891 (2018)
Latorres, J.M., et al.: Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 55(2), 721–729 (2018)
Barse, A.V., et al.: Endocrine disruption and metabolic changes following exposure of Cyprinus carpio to diethyl phthalate. Pestic. Biochem. Physiol. 88(1), 36–42 (2007)
Klomklao, S., et al.: Extraction of carotenoprotein from black tiger shrimp shells with the aid of bluefish trypsin. J. Food Biochem. 33(2), 201–217 (2009)
Sila, A., et al.: Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 148, 445–452 (2014)
Ghelichi, S., Shabanpour, B., Pourashouri, P.: Properties of fish sausages containing common carp (Cyprinus carpio) roe oil and defatted roe protein hydrolysate during refrigerated storage. J. Aquat. Food Prod. Technol. 27(2), 185–199 (2018)
Sasidharan, A., Venugopal, V.: Proteins and co-products from seafood processing discards: their recovery, functional properties and applications. Waste Biomass Valoriz. 11(11), 5647–5663 (2020)
Messina, C.M., et al.: Vitro bioactivity of astaxanthin and peptides from hydrolisates of shrimp (Parapenaeus longirostris) by-products: from the extraction process to biological effect evaluation, as pilot actions for the strategy “from waste to profit.” Mar. Drugs 19(4), 216 (2021)
Wade, N.M., Gabaudan, J., Glencross, B.D.: A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquacult. 9(2), 141–156 (2017)
de Silva, M.P.K.S.K., Senaarachchi, W.A.R.K.: Efficiency of biotransformation of shellfish waste to carotenoprotein by autolysis and crab-shrimp endo-enzymes. J. Aquat. Food Prod. Technol. 30(5), 526–534 (2021)
Cremades, O., et al.: Isolation and characterization of carotenoproteins from crayfish (Procambarus clarkii). Food Chem. 82(4), 559–566 (2003)
Chalamaiah, M., et al.: Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 31(2), 388–398 (2015)
Chalamaiah, M., et al.: Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res. Int. 52(1), 221–229 (2013)
Wu, X.-Y., Yang, Y.-F.: Heavy metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. J. Food Compos. Anal. 24(1), 62–65 (2011)
Author information
Authors and Affiliations
Contributions
PP: supervision, conceptualization, formal analysis, writing—reviewing and editing; HM: methodology, data curation, resources; AK: visualization, investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pourashouri, P., Mirsadeghi, H. & Khodanazary, A. Extracting and Physicochemical Properties of Carotenoprotein from Shrimp Processing Waste by Proteases-Mediated Hydrolysis. Waste Biomass Valor 13, 1169–1178 (2022). https://doi.org/10.1007/s12649-021-01561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01561-4