Abstract
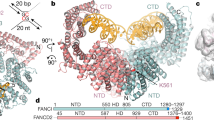
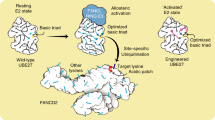
The Fanconi anemia (FA) pathway is essential for the repair of DNA interstrand crosslinks. Central to the pathway is the FA core complex, a ubiquitin ligase of nine subunits that monoubiquitinates the FANCI–FANCD2 (ID) DNA clamp. The 3.1 Å structure of the 1.1-MDa human FA core complex, described here, reveals an asymmetric assembly with two copies of all but the FANCC, FANCE and FANCF subunits. The asymmetry is crucial, as it prevents the binding of a second FANCC–FANCE–FANCF subcomplex that inhibits the recruitment of the UBE2T ubiquitin conjugating enzyme, and instead creates an ID binding site. A single active site then ubiquitinates FANCD2 and FANCI sequentially. We also present the 4.2-Å structures of the human core–UBE2T–ID–DNA complex in three conformations captured during monoubiquitination. They reveal the core–UBE2T complex remodeling the ID–DNA complex, closing the clamp on the DNA before ubiquitination. Monoubiquitination then prevents clamp opening after release from the core.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The coordinates of core, core–ID, core–UBE2T–ID, core–UBE2T–ID–DNA (three conformations) and the corresponding cryo-EM maps, including the focused reconstructions and the composite maps used in refinement, have been deposited at the Protein Data Bank (PDB) and the Electron Microscopy Data Bank (EMDB) under accession codes PDB 7KZP and EMDB-23085 (core complex), PDB 7KZQ and EMDB-23086 (core–ID), PDB 7KZR and EMDB-23087 (core–UBE2T–ID), PDB 7KZS and EMDB-23088 (open-ID conformation core–UBE2T–ID–DNA), PDB 7KZT and EMDB-23089 (intermediate-ID conformation core–UBE2T–ID–DNA) and PDB 7KZV and EMDB-23090 (closed-ID conformation core–UBE2T–ID–DNA).
References
Taylor, A. M. R. et al. Chromosome instability syndromes. Nat. Rev. Dis. Prim. 5, 64 (2019).
Ceccaldi, R., Sarangi, P. & D’Andrea, A. D. The Fanconi anaemia pathway: new players and new functions. Nat. Rev. Mol. Cell Biol. 17, 337–349 (2016).
Zhang, J. et al. DNA interstrand cross-link repair requires replication-fork convergence. Nat. Struct. Mol. Biol. 22, 242–247 (2015).
Lopez-Martinez, D., Liang, C. C. & Cohn, M. A. Cellular response to DNA interstrand crosslinks: the Fanconi anemia pathway. Cell. Mol. Life Sci. 73, 3097–3114 (2016).
Longerich, S., San Filippo, J., Liu, D. & Sung, P. FANCI binds branched DNA and is monoubiquitinated by UBE2T-FANCL. J. Biol. Chem. 284, 23182–23186 (2009).
Yuan, F., El Hokayem, J., Zhou, W. & Zhang, Y. FANCI protein binds to DNA and interacts with FANCD2 to recognize branched structures. J. Biol. Chem. 284, 24443–24452 (2009).
Boisvert, R. A. & Howlett, N. G. The Fanconi anemia ID2 complex: dueling saxes at the crossroads. Cell Cycle 13, 2999–3015 (2014).
Patel, D. R. & Weiss, R. S. A tough row to hoe: when replication forks encounter DNA damage. Biochem. Soc. Trans. 46, 1643–1651 (2018).
Sirbu, B. M. et al. Identification of proteins at active, stalled, and collapsed replication forks using isolation of proteins on nascent DNA (iPOND) coupled with mass spectrometry. J. Biol. Chem. 288, 31458–31467 (2013).
Cole, A. R., Lewis, L. P. & Walden, H. The structure of the catalytic subunit FANCL of the Fanconi anemia core complex. Nat. Struct. Mol. Biol. 17, 294–298 (2010).
Hodson, C., Purkiss, A., Miles, J. A. & Walden, H. Structure of the human FANCL RING-Ube2T complex reveals determinants of cognate E3-E2 selection. Structure 22, 337–344 (2014).
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
van Twest, S. et al. Mechanism of ubiquitination and deubiquitination in the Fanconi anemia pathway. Mol. Cell 65, 247–259 (2017).
Huang, Y. et al. Modularized functions of the Fanconi anemia core complex. Cell Rep. 7, 1849–1857 (2014).
Rajendra, E. et al. The genetic and biochemical basis of FANCD2 monoubiquitination. Mol. Cell 54, 858–869 (2014).
Leung, J. W. et al. Fanconi anemia (FA) binding protein FAAP20 stabilizes FA complementation group A (FANCA) and participates in interstrand cross-link repair. Proc. Natl Acad. Sci. USA 109, 4491–4496 (2012).
Leveille, F. et al. The Fanconi anemia gene product FANCF is a flexible adaptor protein. J. Biol. Chem. 279, 39421–39430 (2004).
Gordon, S. M. & Buchwald, M. Fanconi anemia protein complex: mapping protein interactions in the yeast 2- and 3-hybrid systems. Blood 102, 136–141 (2003).
Pace, P. et al. FANCE: the link between Fanconi anaemia complex assembly and activity. EMBO J. 21, 3414–3423 (2002).
Medhurst, A. L. et al. Evidence for subcomplexes in the Fanconi anemia pathway. Blood 108, 2072–2080 (2006).
Swuec, P. et al. The FA core complex contains a homo-dimeric catalytic module for the symmetric mono-ubiquitination of FANCI-FANCD2. Cell Rep. 18, 611–623 (2017).
Shakeel, S. et al. Structure of the Fanconi anaemia monoubiquitin ligase complex. Nature 575, 234–237 (2019).
Sato, K., Toda, K., Ishiai, M., Takata, M. & Kurumizaka, H. DNA robustly stimulates FANCD2 monoubiquitylation in the complex with FANCI. Nucleic Acids Res. 40, 4553–4561 (2012).
Liang, C. C. et al. The FANCD2–FANCI complex is recruited to DNA interstrand crosslinks before monoubiquitination of FANCD2. Nat. Commun. 7, 12124 (2016).
Longerich, S. et al. Regulation of FANCD2 and FANCI monoubiquitination by their interaction and by DNA. Nucleic Acids Res. 42, 5657–5670 (2014).
Joo, W. et al. Structure of the FANCI–FANCD2 complex: insights into the Fanconi anemia DNA repair pathway. Science 333, 312–316 (2011).
Wang, R., Wang, S., Dhar, A., Peralta, C. & Pavletich, N. P. DNA clamp function of the monoubiquitinated Fanconi anaemia ID complex. Nature 580, 278–282 (2020).
Alcón, P. et al. FANCD2–FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair. Nat. Struct. Mol. Biol. 27, 240–248 (2020).
Polito, D. et al. The carboxyl terminus of FANCE recruits FANCD2 to the Fanconi anemia (FA) E3 ligase complex to promote the FA DNA repair pathway. J. Biol. Chem. 289, 7003–7010 (2014).
Kowal, P., Gurtan, A. M., Stuckert, P., D’Andrea, A. D. & Ellenberger, T. Structural determinants of human FANCF protein that function in the assembly of a DNA damage signaling complex. J. Biol. Chem. 282, 2047–2055 (2007).
Karras, G. I. et al. HSP90 shapes the consequences of human genetic variation. Cell 168, 856–866 (2017).
Adachi, D. et al. Heterogeneous activation of the Fanconi anemia pathway by patient-derived FANCA mutants. Hum. Mol. Genet. 11, 3125–3134 (2002).
Buetow, L. et al. Casitas B-lineage lymphoma linker helix mutations found in myeloproliferative neoplasms affect conformation. BMC Biol. 14, 76 (2016).
Plechanovova, A., Jaffray, E. G., Tatham, M. H., Naismith, J. H. & Hay, R. T. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 (2012).
Pruneda, J. N. et al. Structure of an E3:E2~Ub complex reveals an allosteric mechanism shared among RING/U-box ligases. Mol. Cell 47, 933–942 (2012).
Dou, H., Buetow, L., Sibbet, G. J., Cameron, K. & Huang, D. T. BIRC7–E2 ubiquitin conjugate structure reveals the mechanism of ubiquitin transfer by a RING dimer. Nat. Struct. Mol. Biol. 19, 876–883 (2012).
Smogorzewska, A. et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell 129, 289–301 (2007).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Jones, T. A. Interactive electron-density map interpretation: from INTER to O. Acta Crystallogr. D Biol. Crystallogr. 60, 2115–2125 (2004).
Brown, A. et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D Biol. Crystallogr. 71, 136–153 (2015).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Wijker, M. et al. Heterogeneous spectrum of mutations in the Fanconi anaemia group A gene. Eur. J. Hum. Genet. 7, 52–59 (1999).
Morgan, N. V., Tipping, A. J., Joenje, H. & Mathew, C. G. High frequency of large intragenic deletions in the Fanconi anemia group A gene. Am. J. Hum. Genet. 65, 1330–1341 (1999).
Acknowledgements
We thank the staff of the MSKCC Cryo-EM facility, the NYSBC Simons Electron Microscopy Center (supported by grants from the Simons Foundation (SF349247), NYSTAR and the NIH National Institute of General Medical Sciences (GM103310)) and the HHMI Cryo-EM facility for help with data collection. This work was supported by HHMI and National Institutes of Health grant no. CA008748.
Author information
Authors and Affiliations
Contributions
S.W. carried out the biochemical experiments and collected and analysed the cryo-EM data. R.W. prepared the ID complex. C.P. and A.Y. carried out cell culture and protein purification. N.P.P. and S.W. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Anke Sparmann was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM reconstruction of the FA Core, Core-ID and Core-UBE2T-ID complexes.
a, Left, coomassie stained gel of the purified Core Complex containing recombinant subunits FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FAAP100 and endogenous FAAP20 as labeled. Right, immunoblots confirming the identity of the FAAP20 and also showing the presence of FANCB, which overlaps with FANCG in the coomassie-stained gel. b-d, Flowcharts of single particle cryo-EM data processing of the Core complex (b), Core-ID complex (c) and Core-UBE2T-ID complex (d). Consensus and focused reconstructions are colored by local resolution (RELION3) as indicated with the color keys (“>” marks a low resolution cutoff of ~10 Å). Orientation is similar to Fig. 1a. In b, all seven focused reconstructions are shown individually in orientations that match the consensus map (inside two dashed rectangles). They are also shown in a combined style with their corresponding masks used for focused refinements shown as semi-transparent in different colors (below the green dashed rectangle). The composite map (below the orange dashed rectangle) is shown in semi-transparent rendering and colored by subunit. In c and d, all focused reconstructions are combined in a single figure, with their corresponding masks shown as semi-transparent surfaces in different colors. Overall resolutions for each focused map are also indicated. The composite maps are shown in semi-transparent rendering and colored by subunit. Graphs at the bottom show gold-standard FSC plots between two independently refined half-maps for the consensus and focused reconstructions with the FSC cutoff 0.143 marked by horizontal dashed lines. The FSC plot of the refined models (labeled PDB) versus the composite cryo-EM map is shown in red, with the FSC cutoff marked by vertical dashed lines. The compositions of the masks are described in Supplementary Note 1.
Extended Data Fig. 2 Cryo-EM reconstruction of the three conformations of the Core-UBE2T-ID-DNA complex from the ubiquitination reaction.
a, Flowchart of single particle cryo-EM data processing of the Core-UBE2T-ID-DNA complex in three different conformations. Consensus and focused maps are colored by local resolution and oriented as in Fig. 1b (slight rotations were applied for clarity). Focused reconstructions are shown in a combined view as labeled, with the corresponding masks shown in semi-transparent rendering in different colors. All reconstruction4/mask4 renderings are shown separately. The composite maps are also semi-transparent and are colored by subunit. The four graphs on the right show gold-standard FSC curves between two independently refined half-maps for the consensus and focused reconstructions, with the FSC cutoff 0.143 marked by horizontal dashed lines. b, Left, density attributed to ubiquitin (yellow) extending from UBE2T in the 7.1 Å focused reconstruction (~20 % of the closed-ID Core-UBE2T-ID-DNA Ub+ particles), masked by mask4 of a. This density is absent in the low pass filtered reconstruction of the rest of the particles (right map, Ub-). c, Cartoon representations of models rigid-body fitted into the Ub+ map: Core-UBE2T-ID-DNA in closed-ID conformation (colored as in Fig. 1d), ubiquitin (PDB 4BVU, yellow), and mono-ubiquitinated FANCD2 (gray, Ub orange) from IDUb (PDB 6VAE) superimposed on FANCD2. The ubiquitin C-terminus (“C” in black sphere) is closer to UBE2T Cys86 (labeled) than to FANCD2 Lys561 (labeled), which is at the opposite side of Cys86. d, Superposition of UBE2T-Ub moieties (ubiquitin in yellow) and two other E2-Ub conjugates on UBE2T (ubiquitin from PDB 4AP4 in green, and that from 5TTE in blue, all E2 in magenta). e, Gold-standard FSC plot between two independently refined half-maps for the Ub+ reconstruction, with the FSC cutoff 0.143 marked by horizontal dashed lines.
Extended Data Fig. 3 Conformations and dimerization of FANCB-FAAP100 and FANCA.
a, FANCB-FAAP100 heterodimer colored as in Fig. 1a, except for the FANCB β sandwich (red) and α/β domain (blue), and the FAAP100 β sandwich (orange) and α/β domain (pink). b-c, Superposition of the β sandwich (b) and α/β domains (c) of FANCB and FAAP100. d, Illustration showing the divergent domain-domain packing arrangements of FANCB and FAAP100, aligned within the indicated dotted boxes. e-g, Dimer of FANCB-FAAP100 heterodimer looking down the 2-fold rotation axis (marked red). WD40 domains are rendered with an envelope to facilitate comparison of their different positions and orientations. f-g Superposition of the dimer with a 2-fold rotated copy aligned on the central core, looking down the 2-fold axis (f) or rotated 90° about the axis (g), showing the WD40 and most of the coiled-coil domains do not superimpose. h-i, Superpositions of the coiled-coil helices (h) and the WD40-WD40 pairs (i) at the inactive-side (FANCB blue, FAAP100 green) and active-side (both gray). The helical bend angles and their approximate positions are as shown. Red sticks indicate the internal rotation axis of each WD40 domain; arrows show the directions of the central β strands. j, Side by side comparison of FANCA (orange NTD) and FANCA’ (yellow NTD), superimposed on their CTD domains (both gray). The MD domain (red) is disordered in FANCA’ (dashed circle). k-l, Superpositions of the FANCA and FANCA’ NTD domains (gray) (k) and the N-terminal segments (colored as in j) (l). m, Close-up view of contacts between FANCA’ NTD and the HB domains of FANCB and FAAP100. n, Close-up view of FANCA dimerization interface, colored as in Fig. 1a (black line shows axis of dimer symmetry), showing interacting residues (green-dotted lines indicate hydrogen bonds) (additional discussion of n is in Supplementary Note 2).
Extended Data Fig. 4 Inter-subunit interactions of FANCL and of FANCC-FANCENTD-FANCF.
a-b, Overall views of FANCL at the active-side (a) and inactive-side (b) of the apo-Core complex also showing their Core-contacts. View in b is oriented as in a by superposition on the RWD1 domain. Dashed red circle indicates that the FANCL’ RING domain is poorly ordered in the apo-Core complex structure. The regions that panels d-i zoom into are delineated by dashed rectangles. The N- and C-termini of FANCL/FANCL’ are labeled. c, Top, the FANCC-FANCENTD-FANCF subcomplex also showing FANCL to which it makes its majority of contacts. View is rotated by 62° about the horizontal axis relative to b. Bottom, view rotated by 180° about the vertical axis. The regions that panels j-m zoom into are delineated by dashed rectangles looking into the plane of the figure, or by slanted rectangles with arrows indicating the direction of the view. The N- and C-termini of FANCC, FANCENTD and FANCF are labeled. d-g, Close-up views showing interactions of FANCB-FAAP100 coiled-coil with the FANCL RWD2 domain at the active (d) and inactive (e) sides of the Core complex, with the FANCL RWD1 domain at the active (f) and inactive (g) sides of the Core complex. h-i, Close-up views of the contacts FANCC and FANCENTD make to the FANCL RWD1, RWD3 and RING domains at the inactive side. The helix α18 of FANCC is not shown for clarity in h. j-m, Close-up views of the contacts between FANCC and FANCF (j, k), and between FANCC and FANCENTD (l, m). Subunits are colored as in Fig. 1a. Only side chains involved in intermolecular interactions are shown. Green dotted lines indicate hydrogen bond contacts. Dashed colored curves indicate disordered regions. The interactions shown in panels d to m are described in Supplementary Note 3.
Extended Data Fig. 5 Hypothetical assembly sequence for the Core complex.
a, Individual Core subunits are rendered as low-resolution surfaces and colored as in Fig. 1a. At step 1, two FANCL subunits bind to the hub of a dimer of FANCB-FAAP100 heterodimers. At step 2, FANCC-FANCE-FANCF and a dimer of FANCG-FANCA heterodimers transiently bind to the hub, but do not remain stably bound. At step 3, avidity from an interaction between the FANCG and FANCF subunits of the transiently-bound FANCC-FANCE-FANCF and FANCG-FANCA results in their stable association with the hub. This assembly can bind a second FANCG-FANCA dimer of heterodimers and give rise to a fully symmetric but inactive complex that is found in ~1% of the particles (Extended Data Fig. 7). This symmetric complex is rare because FANCA’-FANCG’ association with the hub in step 4 is intramolecular and presumably faster. The Core could, in principle, transiently bind to additional FANCC-FANCE-FANCF and FANCG-FANCA in step 5, but the FANCF-FANCG bridge that would stabilize their transient association with the hub cannot form owing to the different positions of the FANCB’-FAAP100’ WD40 domains and associated FANCL’ induced by FANCG’ binding. b-c, The FANCA dimer cannot assemble with the hub in a 2-fold symmetric manner. Model of 2-fold symmetric FANCB-FAAP100-FANCL-FANCC-FANCE-FANCF-FANCG with a dimeric FANCA-FANCG subcomplex superimposed on the inactive side of the model in either the inactive-side conformation (FANCG aligned) (b), or in the active side conformation (FANCG’ aligned) (c). The second FANCG of the superimposed subcomplex is in black. Arrow between equivalent atoms indicates the distance (100 Å for b and 220 Å for c) of this second FANCG from the position of FANCG’’ in the 2-fold symmetric model. d, Summary of total surface area buried at subunit-subunit interfaces of the apo-Core and the open and closed ID conformation Core-UBE2T-ID.
Extended Data Fig. 6 Inter-subunit interactions of FANCG.
a-b, Overall views of FANCG (a) or FANCG’(b), oriented similarly by aligning their central region, also showing their binding partners or portions thereof. Dashed rectangles indicate the regions that the subsequent panels zoom into. The N- and C-termini of FANCG, FANCG’, FANCA’, the C-terminus of FANCF and the N-terminus of FANCA are labeled. c-f, Close-up views of the contacts between FANCG and to the N-terminal extended segment of FANCA at the inactive-side (c, d) and active-side (e, f) of the apo-Core complex. They are conserved in both sides, except that the FANCA’ α5 helix is uninvolved in contacts due to the different relative orientation of the subsequent FANCA’ NTD with which it packs (Extended Data Fig. 3j). g, Close-up view of the inactive-side contacts between FANCG and FANCF. Five FANCF residues that when mutated in a cluster compromise Core complex assembly, FANCD2 ubiquitination and ICL resistance30 are marked with a red asterisk. h, Close-up view of the active-side FANCG’ packing with the C-terminal helical repeats of FANCA’. FANCG’ is oriented as in g to underscore the partial overlap in the FANCF and FANCA’ CTD binding sites of FANCG/FANCG’. i, Close-up view of the inactive side FANCG binding to the FAAP100 WD40 domain. The inset shows the FANCG zinc-binding site that is otherwise obscured. j-k, By contrast to the inactive-side FANCG, the active side FANCG’ binds to the FANCB α/β domain (j) and the FANCB’ WD40 domain (k), in two small interfaces. Subunits are colored as in Fig. 1a. Only side chains involved in intermolecular interactions are shown. Green or yellow dotted lines indicate hydrogen bond contacts. The interactions are described in Supplementary Note 4.
Extended Data Fig. 7 An inactive, 2-fold symmetric Core complex corresponding to ~1% of the data set.
a, Graph shows gold-standard FSC plots between two independently refined half-maps for the three focused bodies of the RELION3 multi-body reconstruction in point group C1 with 7,331 particles. The resolution of the three bodies at the FSC value of 0.143 is 6.4, 6.5 and 7.6 Å, with the third body corresponding to what is the active side in the canonical Core complex. b, Model of the proper 2-fold symmetric Core, built by fitting individual subunits or their domains into the density with CHIMERA46. There is residual FANCA density only for its NTD, although it is very weak on the side (right side) that has overall weaker density. It is possible that the stronger FANCANTD density on the left side is due to 3D classification not removing all asymmetric Core particles. View is looking down the 2-fold axis (marked by curved arrow) as in Fig. 1a. c, Superposition of the 2-fold symmetric (colored as in b) and asymmetric (gray) Core complexes by aligning the central portion of the FANCB-FAAP100 hub. d-f, Cryo-EM density of the proper 2-fold symmetric Core complex in four orientations related by operations shown. The partially transparent maps are colored by subunit, and the model is overlaid. d is the same orientation as b. FANCC’-FANCE’-FANCF’-FANCG’-FANCL’ are labeled. The bashed box is the area shown in a close-up in g. g, Close-up view of f focusing on the second FANCL’ sequestered by FANCC’-FANCE’-FANCF’.
Extended Data Fig. 8 Fanconi Anemia missense mutations in the Core complex.
a, Summary of mutations in Core complex subunits from the Fanconi Anemia Mutation Database (http://www2.rockefeller.edu/fanconi/). For each subunit, the total number of database entries, the subset that represents missense mutations, and the missense mutations annotated as pathogenic or likely pathogenic are listed. The overall number of missense mutations is much smaller than other types of alterations. The most prevalent alterations are intragenic deletions in FANCA, suggested to correlate with the number of intronic Alu repeats, as well as microdeletions and insertions at homopolymeric tracts or direct repeats47,48. b, The pathogenic missense mutations are mapped onto the Core-ID-DNA structure, rendered semi-transparent, in an orientation as in Fig. 1a. Residues reported mutated two or more times are shown as spheres with a diameter proportional to the number of mutations in the database, except for those with 2 to 4 mutations, which have the same, smallest diameter. c, Column graph of the number of pathogenic missense mutations in the FANCA protein, with the frequently mutated residues colored by their vicinity to the various structural and functional elements of the protein as indicated. Residues mutated 10 or more times are labeled. d, Overall view of the mutated residues (thick sticks) in the vicinity of the FANCA homodimer interface (semi-transparent cartoon), looking down the approximate 2-fold axis indicated by a meniscus. Inactive-side FANCA mutants are in magenta (labeled), and those of the active-side FANCA’ in ruby. e-h, Close-up views of mutations from d. The mutations are discussed in Supplementary Note 5. i-l, Close-up views of FANCA mutations near the FANCA’ CTD that packs with FANCG’. m-q, Close-up views of other FANCA mutations. r-w, Close-up views of FANCG mutations. x-y, Close-up views of FANCC mutations. z, Close-up views of a FANCE mutation.
Extended Data Fig. 9 FANCI and FANCD2 conservation and conformational changes.
a-c, Molecular surface representations of FANCD2 (a), FANCL’-UBE2T (b) and FANCI (c) viewed from the perspective of the interacting partner (FANCL’-UBE2T perspective for FANCI and FANCD2, and ID perspective for FANCL’-UBE2T). For each, left surface has the intermolecular contacts marked in green (FANCL’UBE2T contacts for FANCI and FANCD2, and ID contacts for FANCL’-UBE2T), and right surface is colored by conservation (yellow to red for low to high conservation). Select elements of FANCL’UBE2T are labeled. Dark curve on FANCL’ delineates its RWD3 and RING domains. d, The FANCI (left, cyan) and FANCD2 (right, pink) proteins from the open conformation Core-ID complex superimposed on the corresponding proteins from the free human ID complex27 (orange FANCI and green FANCD2). FANCECTD is shown as a light blue surface. Additional discussion in Supplementary Note 6. e, Closed conformation FANCI (left, cyan) and FANCD2 (right, pink) proteins superimposed on those of the open-conformation complex (orange FANCI and green FANCD2). Additional discussion in Supplementary Note 6. f, Closed conformation FANCI (left, cyan) and FANCD2 (right, pink) superimposed on the IDUb proteins27 (orange FANCI and green FANCD2). Rotation axis and directions are marked by grey lines and curved arrows. Rotation angles, mono-ubiquitination sites, and the N- and C-termini of FANCI and FANCD2 are labeled.
Extended Data Fig. 10 Comparison of the human and chicken Core complexes.
a, Side by side comparison of the human apo-Core complex reported in this paper (left) and the chicken Core complex reported by Shakeel et al22 (right, PDB:6SRI), oriented approximately as Fig. 1e of the Shakeel et al paper. Subunits of the chicken Core are labeled according to the proposed interpretation in the PDB file and figures of the Shakeel et al study. Because FANCB and FAAP100’ (except their WD40 and coiled-coil domains) could not be distinguished in that study, they are both colored blue and labeled as “B-P100 and B’-P100’ ”. Similarly, FANCC, FANCE and the N-terminal portion of FANCF were not distinguished; they are all colored pink and labeled “C-E”. The FANCF C-terminal portion, for which a crystal structure exists30, is labeled as “F”. The rest of the subunits are colored and labeled as in Fig. 1a here. b, Cartoon shows the chicken Core colored gray for polypeptide chains traced in the same direction as the human complex, and dark blue for those traced in the opposite direction (49 % of residues). c, The FANCG’ polypeptide chains in the human (left) and chicken (right) Core complexes have opposite directions. FANCG’ proteins are represented as tubes colored from blue (N-terminus) to red (C terminus). d, Side-by-side comparison of the portion referred to as “base region” in the Shakeel et al study (human Core left, chicken Core right) labeled and colored as in a. e, Comparison of FANCB-FAAP100 WD40-WD40 domains of the human (1st panel) and chicken (2nd and 3rd panels) core complexes. The dotted arrows indicate the self-rotation axis of the β-propellers, with the arrowheads indicating their orientations. The angles between the two axes are marked. Additional discussion in Supplementary Note 7.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Notes 1–7 and Table 1.
Rights and permissions
About this article
Cite this article
Wang, S., Wang, R., Peralta, C. et al. Structure of the FA core ubiquitin ligase closing the ID clamp on DNA. Nat Struct Mol Biol 28, 300–309 (2021). https://doi.org/10.1038/s41594-021-00568-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00568-8
This article is cited by
-
The DNA-damage kinase ATR activates the FANCD2-FANCI clamp by priming it for ubiquitination
Nature Structural & Molecular Biology (2022)
-
The structure-specific endonuclease complex SLX4–XPF regulates Tus–Ter-induced homologous recombination
Nature Structural & Molecular Biology (2022)
-
The key to the FANCD2–FANCI lock
Nature Structural & Molecular Biology (2022)
-
Fanconi anemia: current insights regarding epidemiology, cancer, and DNA repair
Human Genetics (2022)
-
The emergence of a unified mechanism in the Fanconi anemia pathway
Genome Instability & Disease (2021)