Forest Growth Responses to Drought at Short- and Long-Term Scales in Spain: Squeezing the Stress Memory from Tree Rings
- 1Instituto Pirenaico de Ecología -Consejo Superior de Investigaciones Científicas, Zaragoza, Spain
- 2HAZI Fundazioa, Vitoria-Gasteiz, Spain
- 3Departamento de Sistemas Físicos, Químicos y Naturales, Universidad Pablo de Olavide, Sevilla, Spain
- 4Departamento de Biología Ambiental, Universidad de Navarra, Pamplona, Spain
Drought-triggered declines in forest productivity and associated die-off events have increased considerably due to climate warming in the last decades. There is an increasing interest in quantifying the resilience capacity of forests against climate warming and drought to uncover how different stands and tree species will resist and recover after more frequent and intense droughts. Trees form annual growth rings that represent an accurate record of how forest growth responded to past droughts. Here we use dendrochronology to quantify the radial growth of different forests subjected to contrasting climatic conditions in Spain during the last half century. Particularly, we considered four climatically contrasting areas where dominant forests showed clear signs of drought-induced dieback. Studied forests included wet sites dominated by silver fir (Abies alba) in the Pyrenees and beech (Fagus sylvatica) stands in northern Spain, and drought-prone sites dominated by Scots pine (Pinus sylvestris) in eastern Spain and black pine (Pinus nigra) in the semi-arid south-eastern Spain. We quantified the growth reduction caused by different droughts and assessed the short-and long-term resilience capacity of declining vs. non-declining trees in each forest. In all cases, drought induced a marked growth reduction regardless tree vigor. However, the capacity to recover after drought (resilience) at short- and long-term scales varied greatly between declining and non-declining individuals. In the case of beech and silver fir, non-declining individuals presented greater growth rates and capacity to recover after drought than declining individuals. For Scots pine, the resilience to drought was found to be lower in recent years regardless the tree vigor, but the growth reduction caused by successive droughts was more pronounced in declining than in non-declining individuals. In the black pine forest an extreme drought induced a marked growth reduction in declining individuals when accounting for age effects on growth rates. We demonstrate the potential of tree-ring data to record short- and long-term impacts of drought on forest growth and to quantify the resilience capacity of trees.
Introduction
Climate has rapidly and steeply warmed since the 1980s amplifying water shortage through enhanced evapotranspiration and thus increasing the severity of droughts worldwide (Dai, 2013). These climatic trends have made many forests more vulnerable to drought at a global scale as losses in forest productivity and widespread forest die-off episodes have evidenced (Allen et al., 2010).
Nowadays, there is a growing scientific interest on forest reactions to drought across different biomes to discern which growth features or functional traits better characterize different species-specific responses to these climate extremes (Anderegg et al., 2013, 2015, 2016; Vicente-Serrano et al., 2013; Gazol et al., 2017a). In this sense, annual growth rings provide short- (e.g., years) and long-term (e.g., decades) information on how trees respond to drought (e.g., Anderegg et al., 2015; Camarero et al., 2015b; Gazol and Camarero, 2016; Gazol et al., 2017c). Since dry spells reduce forest productivity and tree growth and often weaken trees by deteriorating their vigor, drought effects on forests may last for several years (Peltier et al., 2016). Recent studies suggest that forest resilience to drought, i.e., the capacity to resist and recover after a drought, mainly depends on drought intensity, but such resilience may also depend on climate types (Zhang et al., 2017), tree species or specific functional traits (Greenwood et al., 2017). Furthermore, tree species and individuals can vary in their strategies to cope with drought (Gazol et al., 2017a,b) modifying forest growth responses to drought, which may be reflected in tree-ring width variability.
Droughts are linked to a wide range of climatic conditions, such as increased mean and maximum air temperatures, which rise evapotranspiration rate, reductions in rainfall, more sunshine, and elevated vapor pressure (De Boeck and Verbeeck, 2011). Therefore, droughts do not always share the same climate conditions and, thus, they can differently impact forests growth across different climates and biomes (Choat et al., 2012; Anderegg et al., 2013). For instance, the 2003 heat wave mainly affected central Europe and it was characterized by abnormally warm temperatures during the growing season resulting in a substantial reduction in forest productivity (Ciais et al., 2005). Conversely, the 1994–1995 and 2005 dry spells mostly affected Mediterranean regions of south-western Europe (e.g., Spain) and they were characterized by unusually low values of precipitation that induced widespread growth decline and triggered forest die-off (Peñuelas et al., 2001; Carnicer et al., 2011; Sánchez-Salguero et al., 2012). On the other hand, the 2012 drought mostly affected eastern and southern Spain and it was initiated by very warm and dry conditions during the previous winter and the early growing season (Camarero et al., 2015b). In any case, these conditions represent particular climatic circumstances that translate into forest growth reductions at different time scales.
Droughts have short- and long-term impacts on forest growth. In a short-term perspective (1–5 years), the comparison of forest growth before, during, and after the drought event using resilience indices provides information on forest recovery after drought (Lloret et al., 2011). However, this approach lacks a long-term (10–30 years) perspective of forest response to drought that can only be detected by comparing growth trends of different tree individuals several years or decades after drought started and considering the cumulative effects of consecutive droughts (Peltier et al., 2016). Long-term growth responses and “legacy effects” may include the cumulative effects of successive droughts and differ in duration and severity depending upon several factors (Anderegg et al., 2015). While some tree individuals or species display a marked resilience capacity and recover from drought after 1–5 years, others might maintain reduced growth rates for decades (Yin and Bauerle, 2017). For example, this is the case of some silver-fir (Abies alba) populations showing dieback in the Spanish Pyrenees due to a pronounced and long-term growth decline which started after the 1986 drought (Camarero et al., 2011; Gazol and Camarero, 2016). Ultimately, intense droughts can induce irreversible growth and vigor loss resulting in tree death (Sánchez-Salguero et al., 2012; Camarero et al., 2015b; Cailleret et al., 2017). Here we define the long-term legacy effects by selecting severe droughts representing “tipping-points” in tree vigor and triggering forest dieback (Camarero et al., 2015b). That is, after the selected droughts there should be a clear difference between declining and non-declining individuals in terms of growth at least one to three decades later.
Here, we use different examples of drought-triggered growth decline from forests experiencing die-off in Spain. Our main goal is to demonstrate how the resilience capacity of trees to withstand drought can vary across individuals of different vigor in forests located near the species' rear edges. Moreover, we assess the long- and short-term negative impacts of drought on radial growth and quantify these responses using different simple approaches. Particularly, we focus on how four tree species with contrasting climatic requirements (A. alba, Fagus sylvatica, Pinus sylvestris, and Pinus nigra) respond to different droughts that have affected temperate, continental and Mediterranean forests in Spain. In order to test whether the observed short- and long-term effects of drought on growth vary across sites we studied four forests in the case of beech.
Materials and Methods
Study Forests, Tree Species, and Climate Conditions
To illustrate the short- and long-term responses of trees to drought we studied forests dominated by four different species with contrasting evolutionary and functional characteristics, including one Eurosiberian hardwood species (F. sylvatica L., beech) and three conifers distributed across the Eurosiberian (A. alba Mill., silver fir; P. sylvestris L., Scots pine) and Mediterranean [P. nigra Arn. subsp. salzmannii (Dunal) Franco, black pine] regions (Table 1). Specifically, we studied: four beech forests (Opakua, Arutz, Eraso, and Lokiz) situated across the southern Basque country (Opakua) and Navarra (the other three sites) in the temperate region of northern Spain, a silver fir forest located in the Spanish Pyrenees where temperate conditions prevail, a Scots pine forest situated in the Iberian System (Corbalán, Teruel) subjected to a continental Mediterranean climate and a black pine subjected to semi-arid conditions in Sierra María (Almeria, south-eastern Spain; Table 1). All study sites are located near the southernmost distribution limit of each species (rear edge) in Europe, which potentially make these tree populations sensitive to water shortage (Figure 1).
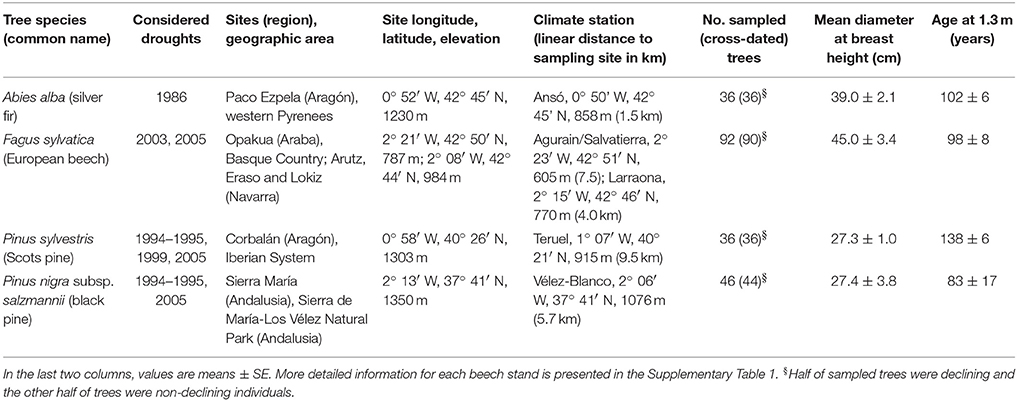
Table 1. Characteristics of the study forests used to quantify tree growth responses to drought across different Spanish regions.
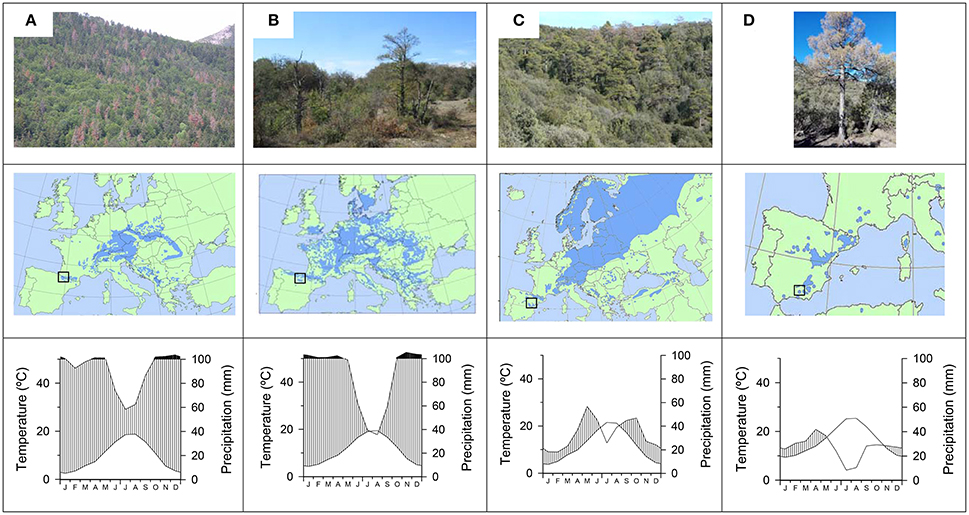
Figure 1. Views, location of study sites within the European distribution area (boxes in the maps), and climate diagrams for the four study tree species showing recent drought-triggered dieback: (A), silver fir; (B) beech, (C) Scots pine; and (D) black pine.
According to monthly climatic data (mean temperatures, precipitation) obtained from nearby local meteorological stations for the period 1950–2016, silver fir and beech occur in the wettest and coolest sites, whereas Scots pine and black pine appear in the driest and warmest sites (Figure 1). In the silver fir site and beech sites, the mean annual temperature is 10.6°C and the annual precipitation is 1175 mm. In the Scots pine and black pine sites the mean annual temperatures are 11.8° and 16.4°C and the annual precipitations are 406 and 322 mm, respectively.
We selected the most severe droughts affecting the study sites using the Standardized Precipitation–Evapotranspiration Index (Vicente-Serrano et al., 2010), which is a multiscalar drought index based on temperature and precipitation data and dependent on the estimated cumulative water balance. Dry and wet conditions correspond to negative and positive SPEI values, respectively. We selected the SPEI duration and month based on previous analyses showing the strongest SPEI-growth relationships in each tree species (Pasho et al., 2011). For instance, these authors found that silver fir growth was strongly related to the 2-months long August SPEI, i.e., the cumulative water deficit from July to August. The SPEI was downloaded for the 1950–2015 period from the SPEI Global Drought Monitor webpage (http://spei.csic.es/map/maps.html) and considering the 0.5° grids including each study site. According to the lowest annual values of this index, the most severe droughts affecting the study areas since the 1980s occurred in 1986, 1994–1995, 1999, 2003, 2005, and 2012 (Table 1; see also Supplementary Figure 1).
Field Sampling and Laboratory Processing
In the field, from 35 to 90 dominant and mature trees were selected in each forest and their size (dbh, diameter at breast height) was measured using tapes. Their percent crown transparency was assessed using binoculars. Trees were classified as declining (crown transparency >50%) and non-declining (crown transparency <50%) individuals according to a visual estimation of their recent defoliation degree (Dobbertin, 2006). In the case of black pine all declining trees showed pronounced needle shedding (crown transparency >80%) or brown foliage in spring 2017 and were dead when they were sampled in autumn.
Dendrochronology was applied to retrospectively quantify the radial growth (ring width) of trees (Fritts, 2001). We used Pressler increment borers to extract two radial cores per tree at 1.3 m and measured ring widths or obtained cross-sections from declining and recently dead trees as in the case of black pine. Wood samples were carefully sanded until ring boundaries were clearly visible, and then the samples were visually cross-dated. Once samples were cross-dated, we used a binocular scope and a LINTAB measuring device (Rinntech, Germany) to measure the tree-ring widths to the nearest 0.01 mm. The accuracy of visual cross-dating was checked with the program COFECHA which calculates moving correlations between each individual series and the mean site series (Holmes, 1983). In each species, half of the trees corresponded to declining individuals and the other half corresponded to non-declining individuals (Table 1).
Tree-ring width series were converted into basal area increment (BAI) which accounts for the geometrical constraint of adding a volume of wood to a stem of increasing radius (Biondi and Qaedan, 2008). We obtained BAI by using the formula:
where rt and rt−1 are the stem radii corresponding to years t and t−1, respectively.
Quantification of Growth Responses to Drought
We quantified the short- and long-term forest growth responses to drought. Short-term responses were quantified by comparing the growth during the drought event (which may last 1–3 years as for instance in the 2003–2005 drought) with the average growth 3 years after and before the drought event. In previous studies we found that a period of 3 years represents a good compromise between drought duration and the short-term growth response (Gazol et al., 2017b). Long-term responses to account for possible legacy effects were quantified by considering growth trends and average growth rates after the drought event. Growth responses to drought were compared between declining vs. non-declining individuals in each site. Linear-mixed effects models (Pinheiro and Bates, 2000) were used to compare the growth before, during and after the drought. Since these measurements represent repeated measures over the same individuals, tree identity was regarded as random factor. The Kruskal-Wallis test was used to compare the growth of declining and non-declining individuals. The long-term BAI trends were quantified using the Kendall tau statistic (τ). Post-hoc analyses based on Tukey's all-pair comparison were applied to test differences in BAI between pre-, drought, and post-drought periods when a significant influence was detected according to the linear mixed-effects models.
In the black pine forest we detected a marked difference in age between younger declining and older non-declining trees (Supplementary Figure 2), thus making difficult the comparison of growth responses to drought based on BAI data. For this reason, we fitted a Generalized Additive Mixed Model (GAMM; Wood, 2006) to the observed BAI data using the temporal trend, tree size, ontogeny, and climate variables (spring precipitation) as predictors. We obtained fitted BAI values for declining and non-declining trees and considering a theoretical age of 50 years and a mean dbh of 30 cm. The model accounted for 42% of the BAI variation in non-declining and declining individuals. Trend analyses were performed on these modeled BAI values.
All analyses were performed in R (R Development Core Team, 2017). The dplR package was used to convert annual ring measures into BAI (Bunn et al., 2016); the nlme package was used to perform linear-mixed effect models (Pinheiro et al., 2014), the multcomp package (Hothorn et al., 2008) was used to perform post-hoc analyses, the stats, and Kendall packages (McLeod, 2011) were used to calculate the Kruskal-Wallis tests and Kendall trends, respectively, and the GAMMs were fitted using the mgcv package (Wood, 2011).
Results
Drought as a Triggering Factor of Forest Die-Off in Silver Fir
The 1986 drought induced a marked growth decline in silver fir (Figure 2A). In the short term, no differences in growth were observed between declining and non-declining individuals 3 years before (F = 1.239, p = 0.272) and after (F = 0.613, p = 0.438) the 1986 drought (Figure 2B). However, growth during the 1986 drought was significantly lower in declining than in non-declining individuals (F = 4.775, p = 0.035). For non-declining individuals, the short-term growth prior to the drought and after the drought were higher than growth during the drought (Z = 3.19, p = 0.001, and Z = 4.60, p = 0.001, respectively). Similar results were obtained for declining individuals (Z = 4.07, p < 0.001, and Z = 5.74, p < 0.001, respectively). Moreover, after the 1986 drought there were marked differences in long-term growth trends (F = 11.40, p = 0.002) between non-declining and declining individuals, which presented an irreversible growth decline (Figure 2C).
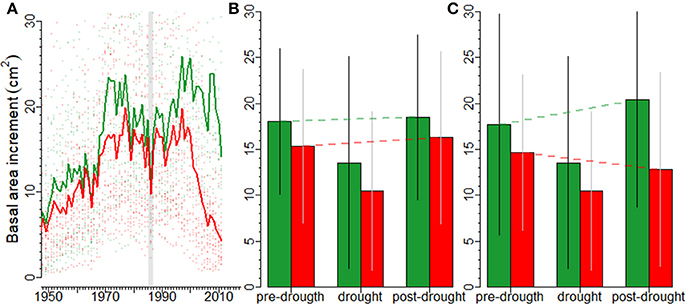
Figure 2. Impacts of drought on forest growth assessed at different time scales in silver fir. Responses of declining (red lines, symbols and bars) and non-declining (green lines, symbols and bars) silver firs to the severe 1986 drought (vertical line); (A) the mean basal area increments (BAI) of declining and non-declining individuals since 1950 and the divergence between the two vigor classes from the 1990s onwards, (B) the short-term pre- (three years before), drought (1986), and post-drought (three years after) BAI values of declining and non-declining individuals are shown. The red and green dashed lines indicate the BAI linear trends from pre- to post-drought growth (resilience; capacity to recover pre-growth levels after drought) in declining and non-declining trees, respectively. Finally, in (C) the long-term pre- (25 years before), drought (1986), and post-drought (25 years after the drought) BAI trends of declining and non-declining individuals are presented. The dashed lines indicate the linear trends from pre- to post-drought BAI (resilience; capacity to recover pre-growth levels after drought. In the figures (B,C) bars show means and standard deviations.
Beech Growth Response to Drought Varies Across Sites
The 2003–2005 drought was characterized by a marked growth reduction in beech growth at the four study sites (Figure 3A). In the short term, we found no significant growth differences between declining and non-declining individuals (Figure 3B; Table 2). In the long-term, post-drought growth differences between declining and non-declining individuals were only detected in Opakua (Figure 3C, Table 2). Declining beech individuals in Eraso presented diminishing growth trends, being significantly lower than non-declining individuals (Table 2). BAI for the 3 years before the dry-warm period was higher than that observed during the dry-warm period (Figure 3B), despite these differences were significant only in Opakua for non-declining individuals and in Arutz for declining individuals (Table 3). Similarly, BAI for this drought was significantly lower than BAI of the three following years in all sites excluding Lokiz for non-declining individuals and Eraso for declining individuals (Figure 3B, Table 3). In the long-term (2006–2017 period), BAI after the 2003–2005 drought significantly increased in non-declining individuals from the Eraso site and also in all individuals from Opakua site (Table 3).
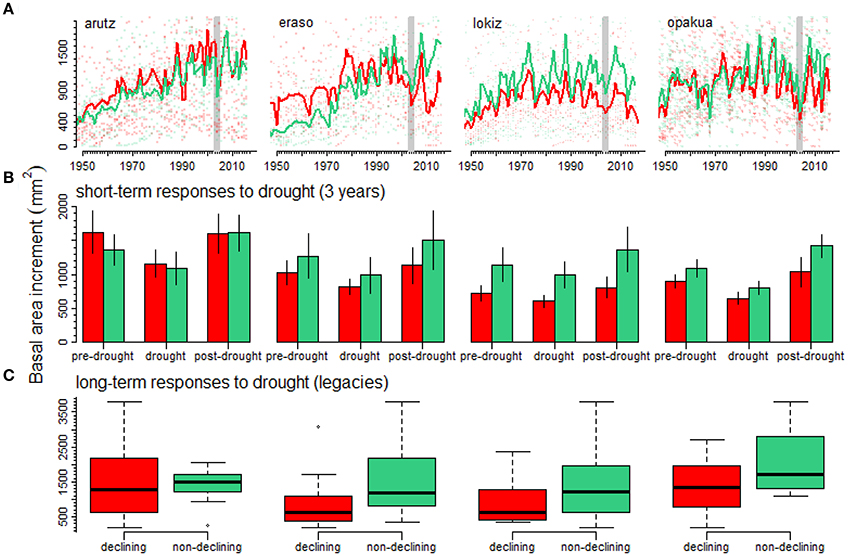
Figure 3. Declining (red lines, symbols and bars) and non-declining (green lines, symbols and bars) beech individuals show different growth responses (A) to drought at short- (B) and long-term (C) scales. Basal area increments are shown for four beech forests (A) separating declining and non-declining individuals. Vertical bars indicate the 2003–2005 drought characterized by warm temperatures and dry conditions that limited beech growth. The short-term response to drought (B) was studied by considering BAI for the three years previous and subsequent to the 2003–2005 drought. The legacy effects of drought on declining and non-declining beech individuals (C) were studied considering the average BAI for the period 2006–2017 following the 2003–2005 drought.
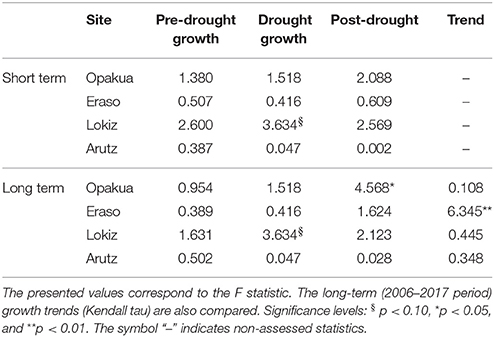
Table 2. Short-term (three-years long) differences in pre- and post-drought growth (basal area increment) values between declining and non-declining beech individuals in the four beech study sites in response to the 2003–2005 drought.
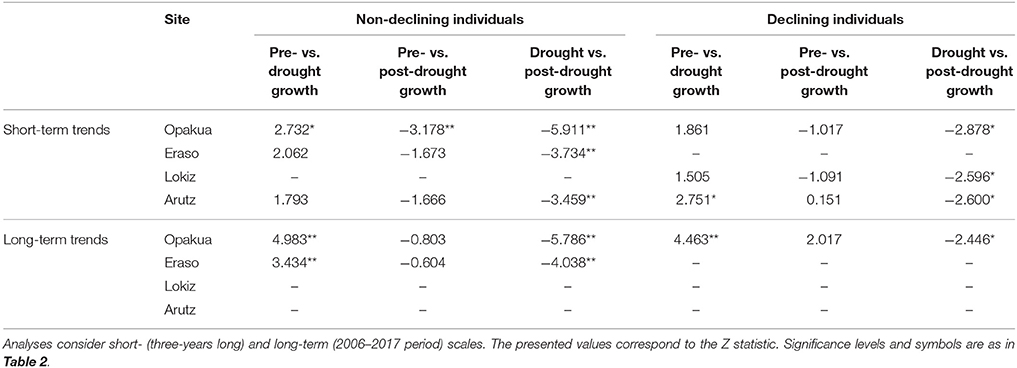
Table 3. Differences between pre- and post-drought growth (basal area increment) observed for declining and non-declining beech individuals in the four study sites in response to the 2003–2005 drought.
Legacy Effects of Drought on Growth in Scots Pine
During the last three decades several droughts have affected the growth of the Scots pine forest studied (Figure 4). An abrupt growth reduction was observed in both declining and non-declining trees in the 1980s. Later, trees had markedly reduced growth during droughts such as those observed in 1994–1995, 1999 and 2005. After the 1980s the BAI trend of non-declining individuals was significantly greater than the BAI trend of declining individuals (Figure 4B; F = 5.86, p = 0.02). The resilience to drought, i.e., the capacity to recover the pre-drought growth values, had decreased for both declining and non-declining trees. However, the rate of decline was higher for declining individuals. In 1994–1995 the post-drought BAI was 1.5 times higher than the pre-drought BAI, whereas in 2005 the ratio between post- and pre-drought BAI was 0.5, meaning that growth had not recovered the pre-drought values (Figure 4C). The differences between pre- and post-drought growth were not significant for the 1994–1995 (F = 2.48, p = 0.128) and 1999 droughts (F = 0.247, p = 0.624). The post-drought BAI after the 2005 drought was marginally significant lower than the pre-drought BAI (F = 3.640, p = 0.059).
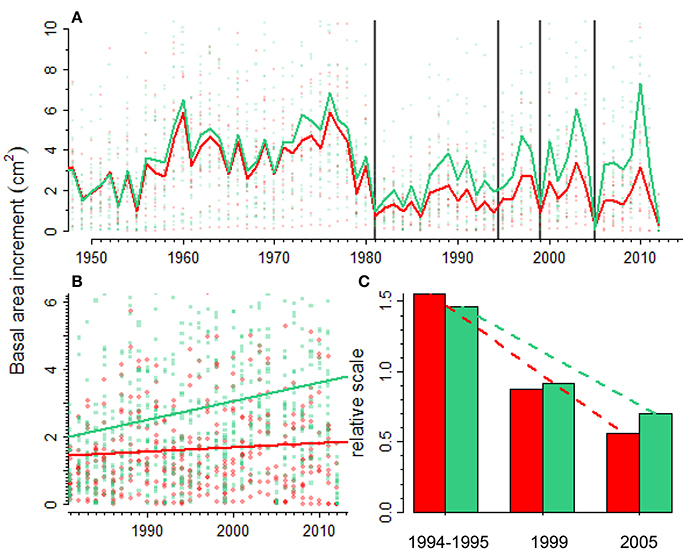
Figure 4. Scots pine growth responses to recurrent droughts (vertical lines). Forest growth (A) is quantified as basal area increment (BAI) and it was reconstructed for declining (red line and symbols) and non-declining (green line and symbols) Scots pine individuals in a forest showing drought-triggered die-off. The difference in BAI between declining and non-declining individuals increases with time since 1980 onwards (B) pointing to the existence of legacy effects on declining individuals. The last figure (C) shows relative growth values (ratio between post- and pre-drought BAI values) for the three most important recent droughts (1994–1995, 1999, and 2005) in declining (red bars) and non-declining (green bars) individuals.
Legacy Effects of Drought on Black Pine Vigor and Growth
Black pine showed a marked decline in growth during the 1994–1995 drought (Figure 5A). Declining trees were significantly younger than non-declining trees (Supplementary Figure 2). To account for this difference in age, we compared BAI values predicted by GAMMs for declining and non-declining individuals and calculated growth trends. It was found a much more negative trend in declining (τ = −0.88) than in non-declining trees (τ = 0.47; see Figure 5C).
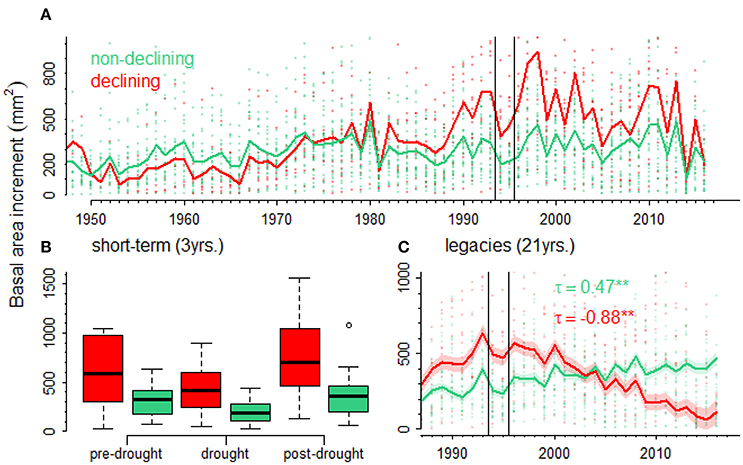
Figure 5. Short- and long-term growth (BAI, basal area increment) responses to drought in declining (red lines, symbols, and bars) and non-declining (green lines, symbols, and bars) black pines. The effect of the 1994–1995 drought (vertical lines delimit BAI values during that period) on growth of declining vs. non-declining black pines is shown (A). Short-term effects of drought on growth (B) were studied by considering the growth three years before (pre-drought), during the drought (drought) and three years after the drought (post-drought). Long-term (legacy) effects (C) were studied by considering growth trends (τ, Kendall tau statistics; significance level, **p < 0.01) of predicted growth values for declining and non-declining trees.
Discussion
Drought Affects Forest Growth at Different Scales
We provide substantial evidence that drought induces a marked decline in forest growth across different forests and tree species. It is well established that water shortage during the growing season and before reduces secondary growth resulting in the formation of narrow rings (Orwig and Abrams, 1997; Fritts, 2001). This has been shown for the study species. For instance, beech growth in northern Spain was reduced during the 2003–2005 drought (Rozas and Olano, 2017). Silver-fir populations in the western Pyrenees were impacted by a severe drought that affected the region in 1986 (Camarero et al., 2011). Iberian Scots pine forest were affected by successive droughts occurring in the early 1980s, 1994–1995, 1999, 2005, and 2012 (Camarero et al., 2015b). Lastly, black pine growth in southern Spain was severely impacted by the 1994–1995 and 2005 droughts (Sánchez-Salguero et al., 2013). However, what currently demands greater attention is to decipher the short- and long-term consequences of drought on forest growth and tree vigor (Anderegg et al., 2015; Peltier et al., 2016; Gazol et al., 2017a). We evidence that drought leads to legacy effects on tree growth and vigor, and that these effects can vary between and within (in the case of beech) tree species. In the short-term, trees are able to recover pre-drought growth rates 3 years after the occurrence of the drought as observed for silver fir (Figure 2B), beech (Figure 3B), and black pine (Figure 5B). However, the long-term impact of drought on forest growth was higher in declining than in non-declining individuals in most cases (Figures 2C, 3C, 5C). Thus, there is also growth “memory” or legacy effects in the long-term recovery capacity after drought as demonstrated by the difference in post-drought growth of non-declining vs. declining Scots pines (Figure 4C). In the long term, drought can have long-lasting effects on forest productivity as demonstrated by the marked reduction in growth of declining silver fir (Figure 2C) and black pine (Figure 5C) individuals. In general, our results agree with published meta-analyses showing that gymnosperms (e.g., pines) often exhibit greater drought legacy than angiosperms (Anderegg et al., 2015), albeit we also detected drought-induced legacy effects on the growth of some beech forests as, for instance, the Eraso site (Figure 3C). Angiosperms have more carbon reserves and display higher maximum hydraulic efficiency than gymnosperms, even if gymnosperms show higher resistance to drought-induced xylem cavitation (Maherali et al., 2004), which allows angiosperms a more rapid growth recovery upon re-watering (Yin and Bauerle, 2017). Discovering how forests will face more frequent and severe droughts remains a challenge but here we provide a framework to advance in our understanding by recovering part of the information contained in tree rings.
We also observed exception to the pattern of declining individuals showing lower growth rates as the case of beech trees in Arutz where non-declining individuals showed lower growth rates (Figure 3A). Crown transparency is commonly used as a measure of tree health status and decline (Camarero et al., 2015b). Moreover, higher growth in declining than in non-declining individuals has been reported in other cases of drought-induced damage (Sangüesa-Barreda et al., 2015). It is plausible to think that under certain circumstances those individuals displaying faster growth rates might be the most vulnerable to drought since they produce wider conduits prone to embolism (Voltas et al., 2013). Moreover, while the pre-drought growth of declining beech individuals in Arutz is higher than that of non-declining individuals it is also true that these differences disappear for the post-drought growth (Figure 3A).
Impacts of Drought on Growth Near the Species Rear Edge
It is expected that species will be more sensitive to climate warming at their rear-edge or southernmost distribution limit (Sánchez-Salguero et al., 2017). In this sense, growth in rear edges is often constrained by low precipitation levels and high evapotranspiration rates enhancing drought stress as shown by previous studies on silver fir, Scots pine, and beech (Jump et al., 2006; Piovesan et al., 2008; Camarero et al., 2011, 2015b). Some silver fir forests in the western Spanish Pyrenees, where die-off events have been reported, show low growth rates compared to European populations subjected to wetter conditions (Gazol et al., 2015). The results provided here clearly show the impact of the 1986 drought on silver-fir forest growth (Figure 2A), which has led to regard that particular drought as a “tipping point” of growth decline and vigor loss in some of these Pyrenean forests (Camarero et al., 2011, 2015b, 2017; Gazol and Camarero, 2016). In a short-term perspective, both declining and non-declining silver fir individuals were able to recover pre-drought growth rates after the 1986 drought (Figure 2B). Nevertheless, when we consider the long-term responses to drought, growth rates of declining individuals decreased and growth values were lower in declining individuals than in non-declining individuals (Figure 2C). This result shows that legacy effects can last for several years after drought (Peltier et al., 2016).
The Iberian system is within the southernmost distribution limit of Scots pine in Europe (Figure 1) and a marked growth decline has been observed in some drought-prone forests of the region (Camarero et al., 2015b; Marqués et al., 2016). In this case, we studied a Scots pine forest in which a severe decline in productivity and a related die-off were observed in response to the severe 2012 drought (Camarero et al., 2015b). The analyses shown here indicate that a severe drought event in the 1980s started a marked growth decline or shift of Scots pine individuals by reducing post-1980s BAI to ca. 25% of pre-1980s values (Figure 4A). The marked drop in growth has been prolonged until today, especially for declining trees (Figure 4B). It is also possible that such sharp growth reduction was magnified by severe outbreaks of the pine processionary moth (Thaumetopoea pityocampa), which affected nearby areas in the 1980s (Sangüesa-Barreda et al., 2014), but we lack precise field information on the study site and growth rings did not show any wood-anatomical evidence of the outbreak. In addition, such growth shift coincides with the onset of the dry-warm 1980s in this area which makes plausible that drought stress was the main cause of the sharp growth drop (Camarero et al., 2015b) and that insect outbreaks contributed to the growth decline. Several droughts have affected the region since the 1980s (1994–1995, 1999, 2005) and the post-drought growth shows that declining individuals have a reduced capacity to recover after drought when compared to non-declining individuals (Figure 4C). Furthermore, multi-year droughts should be considered to evaluate this recovery capacity because they negatively impact forests at several time scales as the 1994–1995 drought did across eastern Spain (Peñuelas et al., 2001). Scots pine and black pine forests in this drought-prone region illustrate this aspect since both species experienced a marked growth reduction in response to the 1994–1995 drought (e.g., Figures 4A, 5A), and they also manifested dieback and high mortality in some regions (Sánchez-Salguero et al., 2012, 2013; Camarero et al., 2015b). Forecasted warmer and drier conditions make some rear-edge Scots pine and black pine populations from eastern Spain highly vulnerable to Twenty-first-century droughts (Sánchez-Salguero et al., 2017).
Long-Term Effects of Drought on Growth: Legacy Effects
Legacy effects tend to be more noticeable in arid or semi-arid biomes such as some xeric rear-edge forests, where growth rates and productivity decrease (Sánchez-Salguero et al., 2012; Vicente-Serrano et al., 2013), compared to wet biomes and more mesic sites (Anderegg et al., 2015; Gazol and Camarero, 2016). Despite all the examples provided here show that drought always causes a marked growth reduction, the magnitude of this growth loss and the associated legacy effects differed considerably between sites. Legacy effects can appear as prolonged periods of productivity decline as a consequence of drought as in the case of the studied silver-fir forest. At this forest, declining and non-declining individuals showed a strong growth reduction during the 1986 drought (Figure 2A). Interestingly, declining individuals displayed a negative long-term growth trend ca. 14 years after the drought event, whereas non-declining individuals showed a positive trend (Figure 2C). In fact, the death of some declining trees could have favored the surviving ones due to a reduction in competition with neighbors (Sangüesa-Barreda et al., 2015). It is plausible that currently declining trees were those showing prior lower growth rates and they were the most vulnerable to the 1986 drought, as indicated their sharp growth reduction in the short term (Figure 2B). These results point to the existence of long-term legacy effects of drought in declining individuals triggered by the 1986 drought which can be considered as a tipping point for growth decline (Camarero et al., 2015b). The understanding of these climatic tipping points should be combined with the assessment of other contributing factors that could promote a differential tree performance, such as past thinning (Camarero et al., 2011, 2017).
Legacy effects can also lead to a reduced capacity to recover after successive droughts (Peltier et al., 2016). This can be the situation observed in the Scots pine forest in which the ratio between post- and pre-drought growth, i.e., the resilience capacity, has declined considerably over three consecutive droughts (Figure 4C). These results suggest that in sight of forecasted more frequent and severe droughts Scots pine populations near the rear-edge may face important long-term productivity declines (Sánchez-Salguero et al., 2017).
In the case of black pine, the apparently lower growth rate of non-declining individuals is an artifact caused by the difference in age between the two groups of trees (Supplementary Figure 1). Ontogenetic factors strongly influence tree growth and may lead to wrong conclusions if not treated appropriately (Bowman et al., 2013). When the BAI data was modeled accounting for the influence of tree age on growth, we found that declining individuals show important legacy effects of drought on growth represented by a negative growth trend after the drought period (Figure 5C). In this case, such negative growth trends could be used to indicate impending tree death (Camarero et al., 2015b; Cailleret et al., 2017).
Short-Term Growth Responses to Drought Vary Across Beech Forests
The 2003–2005 drought caused widespread decline in forest productivity across central Europe (Ciais et al., 2005), and also affected some Mediterranean forests in northern Spain (Camarero et al., 2015a). Several beech populations in northern Spain showed a pronounced growth loss in response to that drought (Rozas et al., 2015). The four forests studied here also showed a marked growth reduction during the 2003–2005 drought (Figure 3A). However, while declining individuals were able to recover pre-drought growth levels, non-declining individuals showed a growth improvement after the drought event (Figures 3B,C). This result evidences the greater recovery capacity of non-declining beech individuals. Moreover, this short-term response was consistent across the four studied forests which are subjected to similar climatic conditions. However, in the long-term the response varied across beech populations (Figure 3C). Non-declining individuals presented higher growth rates than declining individuals after the drought event but these differences were only significant in one of the forests (Opakua; Figure 3C, Table 3). These results suggest that site conditions including topography, soil type or depth, forest composition, stand size and age structures, functional diversity, and past historical use and management (e.g., Camarero et al., 2011) are important factors driving forest responses to drought (Gazol and Camarero, 2016; Gazol et al., 2017b).
Although our study focused on drought stress as a major factor of dieback we cannot discard that other stressors were also involved in these phenomena of vigor loss and mortality. For instance, fungal pathogens (e.g., Hendry et al., 1998), insect outbreaks combined with droughts (Sangüesa-Barreda et al., 2015), or changes in other climatic variables such as increasing vapor pressure deficit due to higher summer temperatures and a reduction in summer fogs in the case of beech (Rozas et al., 2015; Zimmermann et al., 2015) could also be involved on the progressive dieback of some of the study forests.
Conclusions
Assessing the capacity of forests to recover after drought events should be a research priority in sight of their forecasted higher severity. Trees are long-lived organisms that can memorize their past growing conditions in wood. Here we show several cases in which tree rings can be used to understand the effects of drought on tree growth and resilience. Both, short- and long-term tree responses to drought should be considered in order to retrospectively assess forest vulnerability. Regarding our four study cases, we have shown that tree vigor is a component of post-drought resilience as it is demonstrated by the greater capacity to recover after drought of non-declining individuals compared with declining individuals in silver fir and beech. Similarly, long-term legacy effects of drought on forest growth can have different consequences. While in the Scots pine forest, legacy effects are presented as a lower resilience to successive droughts, for the black pine and silver fir forests legacy effects resulted in negative growth trends of declining individuals after the drought event. Long-term monitoring of forest health could complement tree-ring information because the effects of droughts on trees can last for several years and even decades. In addition, dry conditions affect other components of tree growth and functioning not fully recorded by tree-ring data, as for example productivity, leaf and shoot production, fruiting, water, and carbon use.
Author Contributions
JC and AG: lead the writing; AG: performed all analyses; GS-B and AS-M: obtained the tree-ring data; All authors contributed to the discussion and final writing of the manuscript and participated in field sampling.
Funding
Study funded by projects CGL2015-69186-C2-1-R (Spanish Ministry of Economy) and CANOPEE (Interreg V-A POCTEFA 2014-2020, FEDER funds). RS-S is supported by Postdoctoral Grant IJCI-2015-25845.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the support of Jaime de Lara Pasquín (“Sierra María-Los Vélez” Natural Park), A. Oliván (Gob. Aragón), and J.M. Vadillo (Gob. Navarra).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00009/full#supplementary-material
References
Allen, C. D., Macalady, A. K., Chenchouni, H., Bachelet, D., McDowelle, N., Vennetierf, M., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259, 660–684. doi: 10.1016/j.foreco.2009.09.001
Anderegg, L. D. L., Anderegg, W. R. L., and Berry, J. A. (2013). Not all droughts are created equal: translating meteorological drought into woody plant mortality. Tree Physiol. 33, 672–683. doi: 10.1093/treephys/tpt044
Anderegg, W. R. L., Klein, T., Bartlett, M., Sack, L., Pellegrini, A. F., Choat, B., et al. (2016). Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. U.S.A. 113, 5024–5029. doi: 10.1073/pnas.1525678113
Anderegg, W. R. L., Schwalm, C., Biondi, F., Camarero, J. J., Koch, G., Litvak, M., et al. (2015). Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 349, 528–532. doi: 10.1126/science.aab1833
Biondi, F., and Qaedan, F. (2008). A theory-driven approach to tree-ring standardization: defining the biological trend from expected basal area increment. Tree Ring Res. 64, 81–96. doi: 10.3959/2008-6.1
Bowman, D. M., Brienen, R. J., Gloor, E., Phillips, O. L., and Prior, L. D. (2013). Detecting trends in tree growth: not so simple. Trends Plant Sci. 18, 11–17. doi: 10.1016/j.tplants.2012.08.005
Bunn, A., Korpela, M., Biondi, F., Campelo, F., Mérian, P., Qeadan, F., et al. (2016). dplR: Dendrochronology Program Library in R. Avaliable online at: https://CRAN.R-project.org/package=dplR
Cailleret, M., Jansen, S., Robert, E. M. R., Desoto, L., Aakala, T., Antos, J. A., et al. (2017). A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 23, 1675–1690. doi: 10.1111/gcb.13535
Camarero, J. J., Bigler, C., Linares, J. C., and Gil-Pelegrín, E. (2011). Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For. Ecol. Manage. 262, 759–769. doi: 10.1016/j.foreco.2011.05.009
Camarero, J. J., Franquesa, M., and Sangüesa-Barreda, G. (2015a). Timing of drought triggers distinct growth responses in Holm oak: implications to predict warming-induced forest defoliation and growth decline. Forests 6, 1576–1597. doi: 10.3390/f6051576
Camarero, J. J., Gazol, A., Sangüesa-Barreda, G., Oliva, J., and Vicente-Serrano, S. M. (2015b). To die or not to die: early-warning signals of dieback in response to a severe drought. J. Ecol. 103, 44–57. doi: 10.1111/1365-2745.12295
Camarero, J. J., Linares, J. C., Sangüesa-Barreda, G., Sánchez-Salguero, R., Gazol, A., Navarro-Cerrillo, R. M., et al. (2017). “The multiple causes of forest decline in spain: drought, historical logging, competition and biotic stressors,” in Dendroecology: Tree-ring Analyses Applied to Ecological Studies, eds M. M. Amoroso, L. D. Daniels, P. J. Backer, and J. J. Camarero (Cham: Springer International Publishing AG), 307–323.
Carnicer, J., Coll, M., Ninyerola, M., Pons, X., Sánchez, G., and Peñuelas, J. (2011). Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. U.S.A 108, 1474–1478. doi: 10.1073/pnas.1010070108
Choat, B., Jansen, S., Brodribb, T. J., Cochard, H., Delzon, S., Bhaskar, R., et al. (2012). Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. doi: 10.1038/nature11688
Ciais, P., Reichstein, M., Viovy, N., Granier, A., Ogée, J., Allard, V., et al. (2005). Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533. doi: 10.1038/nature03972
Dai, A. (2013). Increasing drought under global warming in observations and models. Nat. Clim. Change 3, 52–58. doi: 10.1038/nclimate1633
De Boeck, H. J., and Verbeeck, H. (2011). Drought-associated changes in climate and their relevance for ecosystem experiments and models. Biogeosciences 8, 1121–1130. doi: 10.5194/bg-8-1121-2011
Dobbertin, M. (2006). Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. Eur. J. For. Res. 125, 89–89. doi: 10.1007/s10342-006-0110-1
Gazol, A., and Camarero, J. J. (2016). Functional diversity enhances silver fir growth resilience to an extreme drought. J. Ecol. 104, 1063–1075. doi: 10.1111/1365-2745.12575
Gazol, A., Camarero, J. J., Anderegg, W. R. L., and Vicente-Serrano, S. M. (2017a). Impacts of droughts on the growth resilience of Northern Hemisphere forests. Glob. Ecol. Biogeogr. 26, 166–176. doi: 10.1111/geb.12526
Gazol, A., Camarero, J. J., Gutiérrez, E., Popa, I., Andreu-Hayles, L., Motta, R., et al. (2015). Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 42, 1150–1162. doi: 10.1111/jbi.12512
Gazol, A., Ribas, M., Gutiérrez, E., and Camarero, J. J. (2017b). Aleppo pine forests from across Spain show drought-induced growth decline and partial recovery. Agric. For. Met. 232, 186–194. doi: 10.1016/j.agrformet.2016.08.014
Gazol, A., Sangüesa-Barreda, G., Granda, E., and Camarero, J. J. (2017c). Tracking the impact of drought on functionally different woody plants in a Mediterranean scrubland ecosystem. Plant Ecol. 218, 1009–1020. doi: 10.1007/s11258-017-0749-3
Greenwood, S., Ruiz-Benito, P., Martínez-Vilalta, J., Lioret, F., Kitzberger, T., Allen, C. D., et al. (2017). Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 20, 539–553. doi: 10.1111/ele.12748
Hendry, S. J., Lonsdale, D., and Boddy, L. (1998). Strip-cankering of beech (Fagus sylvatica): pathology and distribution of symptomatic trees. New Phytol. 140, 549–565. doi: 10.1046/j.1469-8137.1998.00282.x
Holmes, R. L. (1983). Computer-assisted quality control in tree–ring dating and measurement. Tree-Ring Bull. 43, 69–78.
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biom. J. 50, 346–363. doi: 10.1002/bimj.200810425
Jump, A. S., Hunt, J. M., and Peñuelas, J. (2006). Rapid climate-change related growth decline at the southern range edge of Fagus sylvatica. Glob. Change Biol. 12, 2163–2174. doi: 10.1111/j.1365-2486.2006.01250.x
Lloret, F., Keeling, E. G., and Sala, A. (2011). Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120, 1909–1920. doi: 10.1111/j.1600-0706.2011.19372.x
Maherali, H., Pockman, W. T., and Jackson, R. B. (2004). Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85, 2184–2199. doi: 10.1890/02-0538
Marqués, L., Camarero, J. J., Gazol, A., and Zavala, M. A. (2016). Drought impacts on tree growth of two pine species along an altitudinal gradient and their use as early-warning signals of potential shifts in tree species distributions. For. Ecol. Manage. 381, 157–167. doi: 10.1016/j.foreco.2016.09.021
McLeod, A. I. (2011). Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test. R package version 2.2. Avaliable online at https://CRAN.R-project.org/package=Kendall
Orwig, D. A., and Abrams, M. D. (1997). Variation in radial growth responses to drought among species, site, and canopy strata. Trees 11, 474–484. doi: 10.1007/s004680050110
Pasho, E., Camarero, J. J., de Luis, M., and Vicente-Serrano, S. M. (2011). Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 151, 1800–1811. doi: 10.1016/j.agrformet.2011.07.018
Peltier, D. M. P., Fell, M., and Ogle, K. (2016). Legacy effects of drought in the southwestern United States: a multi-species synthesis. Ecol. Monogr. 86, 312–326. doi: 10.1002/ecm.1219
Peñuelas, J., Lloret, F., and Montoya, R. (2001). Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 47, 214–218.
Pinheiro, J. C., and Bates, D. M. (2000). Mixed-Effects Models in S and S-PLUS. New York, NY: Springer-Verlag.
Pinheiro, J., Bates, D., DebRoy, S., and Sarkar, D. (2014). nlme: Linear and Nonlinear Mixed Effects Models. Vienna: R package version 3.1.
Piovesan, G., Biondi, F., Di Fillipo, A., and Maugeri, M. (2008). Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Change Biol. 14, 1265–1281. doi: 10.1111/j.1365-2486.2008.01570.x
R Development Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rozas, V., and Olano, J. M. (2017). Dendroclimatic responses of four European broadleaved tree species near their southwestern range edges. Dendrobiology 77, 65–75. doi: 10.12657/denbio.077.006
Rozas, V., Camarero, J. J., Sangüesa-Barreda, G., Souto, M., and García-González, I. (2015). Summer drought and ENSO-related cloudiness distinctly drive Fagus sylvatica growth near the species rear-edge in northern Spain. Agric. For. Met. 201, 153–164 doi: 10.1016/j.agrformet.2014.11.012
Sánchez-Salguero, R., Camarero, J. J., Dobbertin, M., Fernández-Cancioa, Á., Vilà-Cabrera, A., Manzanedo, R. D., et al. (2013). Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manage. 310, 956–967. doi: 10.1016/j.foreco.2013.09.050
Sánchez-Salguero, R., Camarero, J. J., Gutiérrez, E., González Rouco, F., Gazol, A., Sanguesa-Barreda, et al. (2017). Assessing forest vulnerability to climate warming using a process-based model of tree growth: bad prospects for rear-edges. Glob. Change Biol. 23, 2705–2719. doi: 10.1111/gcb.13541
Sánchez-Salguero, R., Navarro-Cerillo, R. M., Camarero, J. J., and Fernández-Cancio, A. (2012). Selective drought-induced decline of pine species in southeastern Spain. Clim. Change 113, 767–785. doi: 10.1007/s10584-011-0372-6
Sangüesa-Barreda, G., Camarero, J. J., García-Martín, A., Hernández, R., and de la Riva, J. (2014). Remote-sensing and tree-ring based characterization of forest defoliation and growth loss due to the Mediterranean pine processionary moth. For. Ecol. Manage. 320, 171–181. doi: 10.1016/j.foreco.2014.03.008
Sangüesa-Barreda, G., Camarero, J. J., Oliva, J., Montes, F., and Gazol, A. (2015). Past logging, drought and pathogens interact and contribute to forest dieback. Agric. For. Met. 208, 85–94. doi: 10.1016/j.agrformet.2015.04.011
Vicente-Serrano, S. M., Beguería, S., and López-Moreno, J. I. (2010). A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718. doi: 10.1175/2009JCLI2909.1
Vicente-Serrano, S. M., Gouveia, C., Camarero, J. J., Beguería, S., Trigo, R., López-Morenoa, J. I., et al. (2013). Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. U.S.A. 110, 52–57. doi: 10.1073/pnas.1207068110
Voltas, J., Camarero, J. J., Carulla, D., Aguilera, M., Oriz, A., and Ferrio, J. P. (2013). A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ. 36, 1435–1448. doi: 10.1111/pce.12072
Wood, S. N. (2006). Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman and Hall/CRC Press.
Wood, S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 73, 3–36. doi: 10.1111/j.1467-9868.2010.00749.x
Yin, J., and Bauerle, T. L. (2017). A global analysis of plant recovery performance from water stress. Oikos 126, 1377–1388. doi: 10.1111/oik.04534
Zhang, Q., Shao, M., Jia, X., and Wei, X. (2017). Relationship of climatic and forest factors to drought- and heat-induced tree mortality. PLoS ONE 12:e0169770. doi: 10.1371/journal.pone.0169770
Keywords: annual tree rings, black pine, basal area increment, dendroecology, European beech, growth resilience, scots pine, silver fir
Citation: Julio Camarero J, Gazol A, Sangüesa-Barreda G, Cantero A, Sánchez-Salguero R, Sánchez-Miranda A, Granda E, Serra-Maluquer X and Ibáñez R (2018) Forest Growth Responses to Drought at Short- and Long-Term Scales in Spain: Squeezing the Stress Memory from Tree Rings. Front. Ecol. Evol. 6:9. doi: 10.3389/fevo.2018.00009
Received: 12 November 2017; Accepted: 16 January 2018;
Published: 01 February 2018.
Edited by:
Christopher Carcaillet, Ecole Pratique des Hautes Etudes, Université de Sciences Lettres de Paris, FranceReviewed by:
Marina V. Bryukhanova, Siberian Federal University, RussiaBao Yang, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, China
Copyright © 2018 Julio Camarero, Gazol, Sangüesa-Barreda, Cantero, Sánchez-Salguero, Sánchez-Miranda, Granda, Serra-Maluquer and Ibáñez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Julio Camarero, jjcamarero@ipe.csic.es
 J. Julio Camarero
J. Julio Camarero Antonio Gazol
Antonio Gazol Gabriel Sangüesa-Barreda1
Gabriel Sangüesa-Barreda1  Raúl Sánchez-Salguero
Raúl Sánchez-Salguero Elena Granda
Elena Granda Xavier Serra-Maluquer
Xavier Serra-Maluquer Ricardo Ibáñez
Ricardo Ibáñez