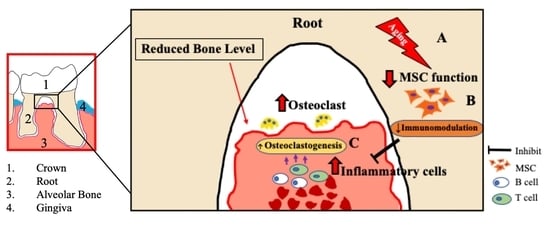
Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model
Abstract
:1. Introduction
2. Results
2.1. Severe Bone Resorption after Ligation in Aged Mice
2.2. Increased Inflammatory Cell Accumulation at the Periodontal Bone Destruction Area in Aged Mice
2.3. Decreased MSCs Number in Aged Mice
2.4. Functional Impairment of Aged MSCs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Periodontitis Model
4.3. Micro CT Analysis
4.4. Histological Analysis
4.5. Immunohistochemical Analysis
4.6. Cell Culture
4.7. Colony-Forming Unit Fibroblastic (CFU-f) Assay
4.8. In Vitro Wound-Healing Assay
4.9. Cell Surface Antigen Analysis
4.10. In Vitro Osteogenic and Adipogenic Differentiation
4.11. Reverse Transcription and Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.12. Senescence Detection Assay
4.13. Isolation, Activation, and Culture of T Cells
4.14. Induction of T Cell Apoptosis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Available online: https://icd.who.int/ (accessed on 23 October 2020).
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [Green Version]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Fortier, L.A. Stem Cells: Classifications, Controversies, and Clinical Applications. Veter Surg. 2005, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Zhang, F.; Tsai, S.; Kato, K.; Yamanouchi, D.; Wang, C.; Rafii, S.; Liu, B.; Kent, K.C. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 17564–17574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Petrini, M.; Pacini, S.; Fazzi, R.; Trombi, L.; Galimberti, S.; Petrini, I. Mesenchymal cells inhibit expansion but not cytotoxicity exerted by gamma-delta T cells. Eur. J. Clin. Investig. 2009, 39, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, I.; Le Blanc, K.; Sundberg, B.; Ringdén, O. Mesenchymal Stem Cells Stimulate Antibody Secretion in Human B Cells. Scand. J. Immunol. 2007, 65, 336–343. [Google Scholar] [CrossRef]
- Traggiai, E.; Volpi, S.; Schena, F.; Gattorno, M.; Ferlito, F.; Moretta, L.; Martini, A. Bone Marrow-Derived Mesenchymal Stem Cells Induce Both Polyclonal Expansion and Differentiation of B Cells Isolated from Healthy Donors and Systemic Lupus Erythematosus Patients. STEM CELLS 2008, 26, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Gonzalez-Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009, 60, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue Engineering of Functional Salivary Gland Tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Developmental Committee of the European Group for, B.; Marrow, T. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [Green Version]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.; Hoshina, H.; Li, M.; Arasawa, M.; Uematsu, K.; Ogawa, S.; Yamada, K.; Kawase, T.; Suzuki, K.; Ogose, A.; et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: Coordinated activation of bone formation and resorption. Bone 2012, 50, 1123–1129. [Google Scholar] [CrossRef]
- Raggi, C.; Berardi, A.C. Mesenchymal stem cells, aging and regenerative medicine. Muscle Ligaments Tendons J. 2012, 2, 239–242. [Google Scholar]
- Bustos, M.L.; Huleihel, L.; Kapetanaki, M.G.; Lino-Cardenas, C.L.; Mroz, L.; Ellis, B.M.; McVerry, B.J.; Richards, T.J.; Kaminski, N.; Cerdenes, N.; et al. Aging Mesenchymal Stem Cells Fail to Protect Because of Impaired Migration and Antiinflammatory Response. Am. J. Respir. Crit. Care Med. 2014, 189, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Bruna, F.; Contador, D.; Conget, P.; Erranz, B.B.; Sossa, C.L.; Arango-Rodríguez, M.L. Regenerative Potential of Mesenchymal Stromal Cells: Age-Related Changes. Stem Cells Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Lopez, E.M.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004. [Google Scholar] [CrossRef] [Green Version]
- Lukjanenko, L.; Karaz, S.; Stuelsatz, P.; Gurriaran-Rodriguez, U.; Michaud, J.; Dammone, G.; Sizzano, F.; Mashinchian, O.; Ancel, S.; Migliavacca, E.; et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 2019, 24, 433–446.e7. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.W.; Wang, Y.-L.; Christensen, J.M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D.S.; Lee, W.A.; Brandacher, G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl. Immunol. 2014, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wei, G.; Guojun, W.; Wen, G.; Qi, B.; Xu, L.; Tao, S. Donor Age and Cell Passage Affect Osteogenic Ability of Rat Bone Marrow Mesenchymal Stem Cells. Cell Biophys. 2015, 72, 543–549. [Google Scholar] [CrossRef]
- Newman, M.G.; Takei, H.H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2019; Volume xxiii, 913p. [Google Scholar]
- Graves, D.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Lu, H.-K.; Chen, Y.-L.; Chang, H.-C.; Li, C.-L.; Kuo, M.Y.-P. Identification of the osteoprotegerin/receptor activator of nuclear factor-kappa B ligand system in gingival crevicular fluid and tissue of patients with chronic periodontitis. J. Periodontal Res. 2006, 41, 354–360. [Google Scholar] [CrossRef]
- Chen, B.; Wu, W.; Sun, W.; Zhang, Q.; Yan, F.; Xiao, Y. RANKL Expression in Periodontal Disease: Where Does RANKL Come from? BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Campbell, L.; Millhouse, E.; Malcolm, J.; Culshaw, S. T cells, teeth and tissue destruction what do T cells do in periodontal disease? Mol. Oral Microbiol. 2015, 31, 445–456. [Google Scholar] [CrossRef]
- Beck, J.D.; Sharp, T.; Koch, G.G.; Offenbacher, S. A 5-year study of attachment loss and tooth loss in community-dwelling older adults. J. Periodontal Res. 1997, 32, 516–523. [Google Scholar] [CrossRef]
- Baelum, V.; Luan, W.-M.; Chen, X.; Fejerskov, O. Predictors of destructive periodontal disease incidence and progression in adult and elderly Chinese. Community Dent. Oral Epidemiol. 1997, 25, 265–272. [Google Scholar] [CrossRef]
- Eke, P.; Dye, B.; Wei, L.; Thornton-Evans, G.; Genco, R. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Luk, F.; Carreras-Planella, L.; Korevaar, S.S.; De Witte, S.F.H.; Borràs, F.E.; Betjes, M.G.H.; Baan, C.C.; Hoogduijn, M.J.; Franquesa, M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front. Immunol. 2017, 8, 1042. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Liang, S.; Hosur, K.B.; Domon, H.; Hajishengallis, G. Periodontal inflammation and bone loss in aged mice. J. Periodontal Res. 2010, 45, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Palmer, R.M. A clinical guide to periodontology: Pathology of periodontal disease. Br. Dent. J. 2014, 216, 457–461. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Morrison, J.; Zadeh, H.H. Comparison of gingival and peripheral blood T cells among patients with periodontitis suggests skewing of the gingival T cell antigen receptor V beta repertoire. J. Periodontal Res. 1999, 34, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.-P.; et al. B and T Lymphocytes Are the Primary Sources of RANKL in the Bone Resorptive Lesion of Periodontal Disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLima, A.J.; Oates, T.; Assuma, R.; Schwartz, Z.; Cochran, D.; Amar, S.; Graves, D.T. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J. Clin. Periodontol. 2001, 28, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Pollina, E.A.; Brunet, A. Epigenetic regulation of ageing: Linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 2015, 16, 593–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, B.; Garinis, G.A.; Hoeijmakers, J.H.J. Age to survive: DNA damage and aging. Trends Genet. 2008, 24, 77–85. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.C.; Tredget, E.E. Concise review: Bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010, 28, 905–915. [Google Scholar]
- Houlihan, D.D.; Mabuchi, Y.; Morikawa, S.; Niibe, K.; Araki, D.; Suzuki, S.; Okano, H.; Matsuzaki, Y. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat. Protoc. 2012, 7, 2103–2111. [Google Scholar] [CrossRef]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochefort, G.Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, N.; Charbord, P.; Eder, V.; Domenech, J. Multipotential Mesenchymal Stem Cells Are Mobilized into Peripheral Blood by Hypoxia. Stem Cells 2006, 24, 2202–2208. [Google Scholar] [CrossRef]
- Iinuma, S.; Aikawa, E.; Tamai, K.; Fujita, R.; Kikuchi, Y.; Chino, T.; Kikuta, J.; McGrath, J.A.; Uitto, J.; Ishii, M.; et al. Transplanted Bone Marrow–Derived Circulating PDGFRα+ Cells Restore Type VII Collagen in Recessive Dystrophic Epidermolysis Bullosa Mouse Skin Graft. J. Immunol. 2015, 194, 1996–2003. [Google Scholar] [CrossRef] [Green Version]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Coipeau, P.; Rosset, P.; Langonne, A.; Gaillard, J.; Delorme, B.; Rico, A.; Domenech, J.; Charbord, P.; Sensebé, L. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy 2009, 11, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Stenderup, K.; Ebbesen, E.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; An, X.; Yang, Y.; Xu, H.; Fan, L.; Deng, L.; Li, T.; Weng, X.; Zhang, J.; Chunhua Zhao, R. MiR-1292 Targets FZD4 to Regulate Senescence and Osteogenic Differentiation of Stem Cells in TE/SJ/Mesenchymal Tissue System via the Wnt/beta-catenin Pathway. Aging Dis. 2018, 9, 1103–1121. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Dewald, H.K.; Martínez-Zamudio, R.I.; Vasilopoulos, T.; Herbig, U.; Fitzgerald-Bocarsly, P. Senescence-associated β-galactosidase activity and other markers of senescence are present in human peripheral blood mononuclear cells during healthy aging. J. Immunol. 2020, 204, 154.15. [Google Scholar]
- Hohlbaum, A.M.; Gregory, M.S.; Ju, S.-T.; Marshak-Rothstein, A. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J. Immunol. 2001, 167, 6217–6224. [Google Scholar] [CrossRef] [Green Version]
- Pluchino, S.; Zanotti, L.; Rossi, B.; Brambilla, E.; Ottoboni, L.; Salani, G.; Martinello, M.; Cattalini, A.; Bergami, A.; Furlan, R.; et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nat. Cell Biol. 2005, 436, 266–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Zhang, L.; Roberts, A.I.; Shi, Y. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J. Immunol. 2008, 181, 190–196. [Google Scholar] [CrossRef]
- Kilpinen, L.; Tigistu-Sahle, F.; Oja, S.; Greco, D.; Parmar, A.; Saavalainen, P.; Nikkilä, J.; Korhonen, M.; Lehenkari, P.; Käkelä, R.; et al. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J. Lipid Res. 2012, 54, 622–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaboosaya, B.; Wulansari, L.K.; Aoki, K.; Kasugai, S. Ligation Period Required to Induce Periodontitis in Mice: Analysis with Micro-computed Tomography. J. Oral Tissue Eng. 2017, 15, 25–34. [Google Scholar]
- Caroti, C.M.; Ahn, H.; Salazar, H.F.; Joseph, G.; Sankar, S.B.; Willett, N.J.; Wood, L.B.; Taylor, W.R.; Lyle, A.N. A Novel Technique for Accelerated Culture of Murine Mesenchymal Stem Cells that Allows for Sustained Multipotency. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hara, E.S.; Ono, M.; Eguchi, T.; Kubota, S.; Pham, H.T.; Sonoyama, W.; Tajima, S.; Takigawa, M.; Calderwood, S.K.; Kuboki, T. miRNA-720 Controls Stem Cell Phenotype, Proliferation and Differentiation of Human Dental Pulp Cells. PLoS ONE 2013, 8, e83545. [Google Scholar] [CrossRef] [Green Version]
- Komori, T.; Ono, M.; Hara, E.S.; Ueda, J.; Nguyen, H.T.T.; Yonezawa, T.; Maeba, T.; Kimura-Ono, A.; Takarada, T.; Momota, R.; et al. Type IV collagen α6 chain is a regulator of keratin 10 in keratinization of oral mucosal epithelium. Sci. Rep. 2018, 8, 2612. [Google Scholar] [CrossRef] [Green Version]







| Gene | GenBank Registration Number | Primer Set | PCR Product Length (bp) | |
|---|---|---|---|---|
| s29 | NM_009093 | Sense: | 5’-GGAGTCACCCACGGAAGTTCGG-3’ | 108 |
| Anti-sense: | 5’-GGAAGCACTGGCGGCACATG-3’ | |||
| Alp | JO2980 | Sense: | 5’-GCTCTCCCTACCGACCCTGTTC-3’ | 130 |
| Anti-sense: | 5’-TGCTGGAAGTTGCCTGGACCTC-3’ | |||
| Ocn | BC055868 | Sense: | 5’-CCAAGCAGGAGGGCAATAAGGTAG-3’ | 122 |
| Anti-sense: | 5’-CTCGTCACAAGCAGGGTCAAGC-3’ | |||
| Lpl | BC003305 | Sense: | 5’-GTCTGGCTGACACTGGACAA-3’ | 221 |
| Anti-sense: | 5’-TGGGCCATTAGATTCCTCAC-3’ | |||
| Pparγ | NM_011146 | Sense: | 5’-GCCAAGGTGCTCCAGAAGATGA-3’ | 155 |
| Anti-sense: | 5’-CGGGTGGGACTTTCCTGCTAAT-3’ | |||
| Tnf-α | BC117057 | Sense: | 5’-GTGGAACTGGCAGAAGAG-3’ | 96 |
| Anti-sense: | 5’-CACAAGCAGGAATGAGAAGA-3’ | |||
| Il1-β | M15131 | Sense: | 5’-AATCTCACAGCAGCACATC-3’ | 194 |
| Anti-sense: | 5’-CCAGCAGGTTATCATCATCAT-3’ | |||
| Ifn-γ | BC119065 | Sense: | 5’-AGCTCATCCGAGTGGTCC-3’ | 88 |
| Anti-sense: | 5’-CTCTTCCCCACCCCGAAT-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, K.T.; Akiyama, K.; Kunitomo, M.; Mun, A.Y.; Tosa, I.; Nguyen, H.T.T.; Zhang, J.; Kohno, T.; Ono, M.; Hara, E.S.; et al. Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model. Int. J. Mol. Sci. 2020, 21, 8103. https://doi.org/10.3390/ijms21218103
Aung KT, Akiyama K, Kunitomo M, Mun AY, Tosa I, Nguyen HTT, Zhang J, Kohno T, Ono M, Hara ES, et al. Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model. International Journal of Molecular Sciences. 2020; 21(21):8103. https://doi.org/10.3390/ijms21218103
Chicago/Turabian StyleAung, Kyaw Thu, Kentaro Akiyama, Masayoshi Kunitomo, Aung Ye Mun, Ikue Tosa, Ha Thi Thu Nguyen, Jiewen Zhang, Teisaku Kohno, Mitsuaki Ono, Emilio Satoshi Hara, and et al. 2020. "Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model" International Journal of Molecular Sciences 21, no. 21: 8103. https://doi.org/10.3390/ijms21218103