Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development
Abstract
:1. Introduction
2. Results
2.1. Perturbation of AtCuAOδ Expression Has a Weak but Significant Effect on Leaf Senescence
2.2. Bolting and Flowering Time Are Affected by Perturbation of AtCuAOδ Expression
2.3. Mutation of AtCuAOδ Results in Delayed Seed Germination and Leaf Emergence While Over-Expression Has Fewer Effects
2.4. PA Content Is Perturbed in AtCuAOδ Mutants and Treatment of WT with the Copper-Containing Amine Oxidase Inhibitor, Aminoguanidine Hydrochloride, Mimics the AtCuAOδ Mutant Germination Phenotype
2.5. Treatment with GA Reverses the Effects of AtCuAOδ Mutation on Seed Germination, Bolting Time and Inflorescence Stem Length
2.6. Endogenous Levels of GAs Are Reduced in AtCuAOδ Mutants But Auxin Level Is Not Consistently Reduced
2.7. Expression of GA Biosynthetic Genes Is Reduced in AtCuAOδ Mutants
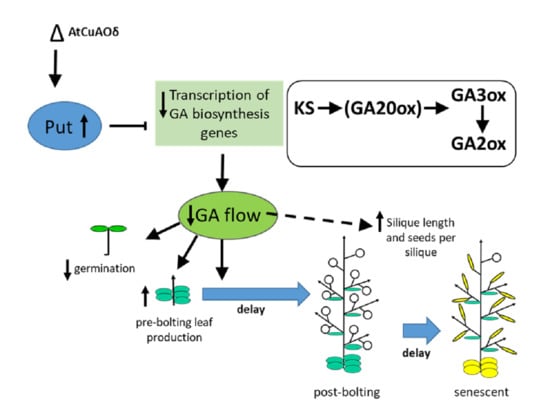
3. Discussion
4. Materials and Methods
4.1. AtCuAOδ Over-Expressors and Insertional Mutants
4.2. Plant Growth Conditions and Germination Assays
4.3. Treatments with GA and Aminoguanidine Hydrochloride
4.4. Dark Induced Senescence and Analysis of Chlorophyll
4.5. PA Analysis
4.6. Real Time PCR
4.7. Analysis of Endogenous Hormones
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Biosynthesis, Signal Transduction and Action, 3rd ed.; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Hanfrey, C.; Sommer, S.; Mayer, M.J.; Burtin, D.; Michael, A.J. Arabidopsis polyamine biosynthesis: Absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J. 2001, 27, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Michael, A.J. Biosynthesis of polyamines in eukaryotes, archaea, and bacteria. In Polyamines; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–14. [Google Scholar]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7, 824–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, R.; Tisi, A.; Rea, G.; Chen, M.M.; Botta, M.; Federico, R.; Cona, A. Involvement of polyamine oxidase in wound healing. Plant Physiol. 2008, 146, 162–177. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Moriguchi, T. Role of polyamines in stress response in horticultural crops. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 35–45. [Google Scholar]
- Galston, A.W.; Kaur-Sawhney, R.; Altabella, T.; Tiburcio, A.F. Plant polyamines in reproductive activity and response to abiotic stress. Bot. Acta 1997, 110, 197–207. [Google Scholar] [CrossRef]
- Hamasaki, N.; Galston, A.W. The polyamines of Xanthium strumarium and their response to photoperiod. Photochem. Photobiol. 1990, 52, 181–186. [Google Scholar] [CrossRef]
- Havelange, A.; Lejeune, P.; Bernier, G.; Kaur-Sawhney, R.; Galston, A.W. Putrescine export from leaves in relation to floral transition in Sinapis alba. Physiol. Plant. 1996, 96, 59–65. [Google Scholar] [CrossRef]
- Applewhite, P.B.; Kaur-Sawhney, R.; Galston, A.W. A role for spermidine in the bolting and flowering of Arabidopsis. Physiol. Plant. 2000, 108, 314–320. [Google Scholar] [CrossRef]
- Alcazar, R.; Garcia-Martinez, J.L.; Cuevas, J.C.; Tiburcio, A.F.; Altabella, T. Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. Plant J. 2005, 43, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Trobacher, C.P.; Shelp, B.J. Arabidopsis aldehyde dehydrogenase 10 family members confer salt tolerance through putrescine-derived 4-aminobutyrate (GABA) production. Sci. Rep. 2016, 6, 35115. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unpredicted proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An ‘electronic fluorescent pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef]
- Fraudentali, I.; Ghuge, S.A.; Carucci, A.; Tavladoraki, P.; Angelini, R.; Cona, A.; Rodrigues-Pousada, R.A. The copper amine oxidase AtCuAO participates in abscisic acid-induced stomatal closure in Arabidopsis. Plants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Huffaker, A.; Pearce, G.; Ryan, C.A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA 2006, 103, 10098–10103. [Google Scholar] [CrossRef] [Green Version]
- Noodén, L.D.; Penney, J.P. Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J. Exp. Bot. 2001, 52, 2151–2159. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Lin, J.-F.; Wu, S.-H.; Swidzinski, J.; Ishizaki, K.; et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef]
- Schippers, J.H.M. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Walters, D. Resistance to plant pathogens: Possible roles for free polyamines and polyamine catabolism. New Phytol. 2003, 159, 109–115. [Google Scholar] [CrossRef]
- Bagni, N.; Ruiz-Carrasco, K.; Franceschetti, M.; Fornalè, S.; Fornasiero, R.B.; Tassoni, A. Polyamine metabolism and biosynthetic gene expression in Arabidopsis thaliana under salt stress. Plant Physiol. Biochem. 2006, 44, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Miura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Clay, N.K.; Nelson, T. Arabidopsis thick vein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport. Plant Physiol. 2005, 138, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Knott, J.M.; Römer, P.; Sumper, M. Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett. 2007, 581, 3081–3086. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Ge, C.; Wang, R.; Wang, H.; Chen, W.; Fu, Z.; Jiang, X.; Li, J.; Wang, Y. The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res. 2010, 20, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.J.; O’Neill, D.P.; Smith, J.J.; Kerckhoffs, L.H.; Elliott, R.C. Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 2000, 21, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Picciarelli, P.; Lombardi, L.; Ceccarelli, N. Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J. Plant Growth Regul. 2011, 30, 405–415. [Google Scholar] [CrossRef]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis lifecycle. Plant J. 2008, 53, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Eriksson, S.; Powers, S.J.; Gong, F.; Griffiths, J.; Woolley, L.; Benlloch, R.; Nilsson, O.; Thomas, S.G.; Hedden, P.; et al. Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 2008, 20, 2420–2436. [Google Scholar] [CrossRef] [Green Version]
- Devaiah, B.N.; Madhuvanthi, R.; Karthikeyan, A.S.; Raghothama, K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2009, 2, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Shevyakova, N.I.; Rakitin, V.Y.; Stetsenko, L.A.; Aronova, E.E.; Kuznetsov, V.V. Oxidative stress and fluctuations of free and conjugated polyamines in the halophyte Mesembryanthemum crystallinum L. under NaCl salinity. Plant Growth Regul. 2006, 50, 69–78. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids—Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Marcé, M.; Brown, D.S.; Capell, T.; Figueras, X.; Tiburcio, A.F. Rapid high-performance liquid chromatographic method for the quantitation of polyamines as their dansyl derivatives: Application to plant and animal tissues. J. Chromatogr. 1995, 666, 329–335. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fambrini, M.; Mariotti, L.; Parlanti, S.; Salvini, M.; Pugliesi, C.A. A GRAS-like gene of sunflower (Helianthus annuus L.) alters the gibberellin content and axillary meristem outgrowth in transgenic Arabidopsis plants. Plant Biol. 2015, 17, 1123–1134. [Google Scholar] [CrossRef]
- Paparelli, E.; Parlanti, S.; Gonzali, S.; Novi, G.; Mariotti, L.; Ceccarelli, N.; van Dongen, J.T.; Kölling, K.; Zeeman, S.C.; Perata, P. Nighttime sugar starvation orchestrates gibberellin biosynthesis and plant growth in Arabidopsis. Plant Cell 2013, 25, 3760–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignolli, F.; Mariotti, L.; Lombardi, L.; Vidoz, M.L.; Ceccarelli, N.; Picciarelli, P. Tomato fruit development in the auxin-resistant dgt mutant is induced by pollination but not by auxin treatment. J. Plant Phys. 2012, 169, 1165–1172. [Google Scholar] [CrossRef]
- Fambrini, M.; Mariotti, L.; Parlanti, S.; Picciarelli, P.; Salvini, M.; Ceccarelli, N.; Pugliesi, C. The extreme dwarf phenotype of the GA-sensitive mutant of sunflower, dwarf2, is generated by a deletion in the ent-kaurenoic acid oxidase1 (HaKAO1) gene sequence. Plant Mol. Biol. 2011, 75, 431–450. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, B.; Hunt, J.D.; Dimitrova, S.; Spadafora, N.D.; Cort, A.P.; Colombo, D.; Müller, C.T.; Ghuge, S.A.; Davoli, D.; Cona, A.; et al. Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development. Int. J. Mol. Sci. 2020, 21, 7789. https://doi.org/10.3390/ijms21207789
Alharbi B, Hunt JD, Dimitrova S, Spadafora ND, Cort AP, Colombo D, Müller CT, Ghuge SA, Davoli D, Cona A, et al. Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development. International Journal of Molecular Sciences. 2020; 21(20):7789. https://doi.org/10.3390/ijms21207789
Chicago/Turabian StyleAlharbi, Basmah, Julie D. Hunt, Simone Dimitrova, Natasha D. Spadafora, Alex P. Cort, Davide Colombo, Carsten T. Müller, Sandip A. Ghuge, Daniela Davoli, Alessandra Cona, and et al. 2020. "Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development" International Journal of Molecular Sciences 21, no. 20: 7789. https://doi.org/10.3390/ijms21207789