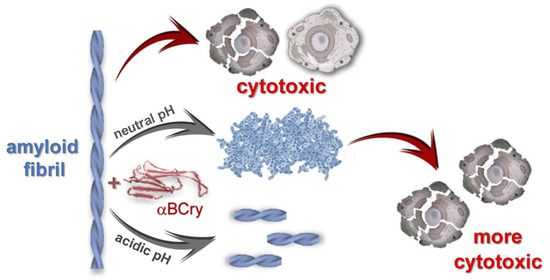
Alpha-B-Crystallin Effect on Mature Amyloid Fibrils: Different Degradation Mechanisms and Changes in Cytotoxicity
Abstract
:1. Introduction
2. Results
2.1. The Studied Amyloid Fibrils Formed from Lysozyme and Beta-2-Microglobulin Have A Similar Structure and Morphology, But Differ in the Size of Their Clusters
2.2. Alpha-B-Crystallin Binds to the Entire Surface of the Amyloid Fiber
2.3. Alpha-B-Crystallin Induces Degradation of Mature Amyloid Fibrils with the Formation of Large Less Ordered Aggregates
2.4. The Rate of Amyloid Fibrils Degradation Induced by Alpha-B-Crystallin Depends to A Greater Extent on Their Thickness and Ability to Cluster Than on the Amino Acid Sequence of the Amyloidogenic Protein
2.5. The Effect of Alpha-B-Crystallin on Amyloid Fibrils Does Not Change Their Resistance to Degradation
2.6. The Alpha-B-Crystallin Action on Amyloid Fibrils Can Reduce Cell Viability in the In Vitro System
2.7. External Influences Can Lead to a Change in The Mechanism of Amyloid Fibrils Degradation Induced by Alpha-B-Crystallin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Alpha-B-Crystallin Isolation and Purification
4.3. Amyloid Fibrils Preparation
4.4. Amyloid Fibrils Degradation by Alpha-B-Crystallin
4.5. Transmission Electron Microscopy
4.6. Confocal Microscopy
4.7. Spectral Measurements
4.8. MTT Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recchia, A.; Debetto, P.; Negro, A.; Guidolin, D.; Skaper, S.D.; Giusti, P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004, 18, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.X.; Qiang, W.; Yau, W.M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 2013, 154, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Naiki, H.; Okoshi, T.; Ozawa, D.; Yamaguchi, I.; Hasegawa, K. Molecular pathogenesis of human amyloidosis: Lessons from β2 -microglobulin-related amyloidosis. Pathol. Int. 2016, 66, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.-I.; Merlini, G.; Saraiva, M.J.M.; Westermark, P. Amyloid fibril proteins and amyloidosis: Chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016, 23, 209–213. [Google Scholar] [CrossRef]
- Esposito, G.; Michelutti, R.; Verdone, G.; Viglino, P.; Hernandez, H.; Robinson, C.V.; Amoresano, A.; Dal Piaz, F.; Monti, M.; Pucci, P.; et al. Removal of the N-terminal hexapeptide from human beta2-microglobulin facilitates protein aggregation and fibril formation. Prot. Sci. Publ. Protein Soc. 2000, 9, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Sattianayagam, P.T.; Gibbs, S.D.J.; Rowczenio, D.; Pinney, J.H.; Wechalekar, A.D.; Gilbertson, J.A.; Hawkins, P.N.; Lachmann, H.J.; Gillmore, J.D. Hereditary lysozyme amyloidosis–phenotypic heterogeneity and the role of solid organ transplantation. J. Intern. Med. 2011, 272, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid – from bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.S.; Balch, W.E.; Kelly, J.W. Functional Amyloid Formation within Mammalian Tissue. PLoS Biol. 2005, 4, e6. [Google Scholar] [CrossRef]
- Kenney, J.M.; Knight, D.; Wise, M.J.; Vollrath, F. Amyloidogenic nature of spider silk. JBIC J. Biol. Inorg. Chem. 2002, 269, 4159–4163. [Google Scholar] [CrossRef]
- Hou, F.; Sun, L.; Zheng, H.; Skaug, B.; Jiang, Q.-X.; Chen, Z.J. MAVS Forms Functional Prion-like Aggregates to Activate and Propagate Antiviral Innate Immune Response. Cell 2011, 146, 841. [Google Scholar] [CrossRef] [Green Version]
- Bieler, S.; Estrada, L.; Lagos, R.; Baeza, M.; Castilla, J.; Soto, C. Amyloid Formation Modulates the Biological Activity of a Bacterial Protein. J. Biol. Chem. 2005, 280, 26880–26885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, M.R.; Valpuesta, J.M. Hsp70 chaperone: A master player in protein homeostasis. F1000Research 2018, 7, 1497. [Google Scholar] [CrossRef] [Green Version]
- Saibil, H.R. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Ganea, E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr. Protein Pept. Sci. 2001, 2, 205–225. [Google Scholar] [CrossRef]
- Plotegher, N.; Kumar, D.; Tessari, I.; Brucale, M.; Munari, F.; Tosatto, L.; Belluzzi, E.; Greggio, E.; Bisaglia, M.; Capaldi, S.; et al. The chaperone-like protein 14-3-3eta interacts with human alpha-synuclein aggregation intermediates rerouting the amyloidogenic pathway and reducing alpha-synuclein cellular toxicity. Hum. Mol. Genetics 2014, 23, 5615–5629. [Google Scholar] [CrossRef] [Green Version]
- Aprile, F.A.; Arosio, P.; Fusco, G.; Chen, S.W.; Kumita, J.R.; Dhulesia, A.; Tortora, P.; Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M.; et al. Inhibition of α-Synuclein Fibril Elongation by Hsp70 Is Governed by a Kinetic Binding Competition between α-Synuclein Species. Biochemistry 2017, 56, 1177–1180. [Google Scholar] [CrossRef]
- Shammas, S.L.; Waudby, C.A.; Wang, S.; Buell, A.K.; Knowles, T.P.; Ecroyd, H.; Welland, M.E.; Carver, J.A.; Dobson, C.M.; Meehan, S. Binding of the molecular chaperone alphaB-crystallin to Abeta amyloid fibrils inhibits fibril elongation. Biophys. J. 2011, 101, 1681–1689. [Google Scholar] [CrossRef] [Green Version]
- Rekas, A.; Jankova, L.; Thorn, D.C.; Cappai, R.; Carver, J.A. Monitoring the prevention of amyloid fibril formation by α-crystallin. FEBS J. 2007, 274, 6290–6304. [Google Scholar] [CrossRef]
- Wälti, M.A.; Schmidt, T.; Murray, D.T.; Wang, H.; Hinshaw, J.E.; Clore, G.M. Chaperonin GroEL accelerates protofibril formation and decorates fibrils of the Het-s prion protein. Proc. Natl. Acad. Sci. USA 2017, 114, 9104–9109. [Google Scholar]
- Yerbury, J.J.; Poon, S.; Meehan, S.; Thompson, B.; Kumita, J.R.; Dobson, C.M.; Wilson, M.R. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007, 21, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Hatters, D.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Howlett, G.J. Suppression of apolipoprotein C-II amyloid formation by the extracellular chaperone, clusterin. JBIC J. Biol. Inorg. Chem. 2002, 269, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Friesen, E.L.; De Snoo, M.L.; Rajendran, L.; Kalia, L.V.; Kalia, S.K. Chaperone-Based Therapies for Disease Modification in Parkinson’s Disease. Park. Dis. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieschke, J.; Cohen, E.; Murray, A.; Dillin, A.; Kelly, J.W. A kinetic assessment of the C. elegans amyloid disaggregation activity enables uncoupling of disassembly and proteolysis. Protein Sci. 2009, 18, 2231–2241. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.G.; Wisen, S.; Gestwicki, J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.N.; Solomon, J.P.; Wang, Y.-J.; Balch, W.E.; Kelly, J.W. Discovery and characterization of a mammalian amyloid disaggregation activity. Protein Sci. 2010, 19, 836–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.N.; Palhano, F.L.; Bieschke, J.; Kelly, J.W. Surface adsorption considerations when working with amyloid fibrils in multiwell plates and Eppendorf tubes. Protein Sci. 2013, 22, 1531–1541. [Google Scholar] [CrossRef] [Green Version]
- Duennwald, M.L.; Echeverria, A.; Shorter, J. Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol. 2012, 10, e1001346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, B.; Ban, T.; Sakai, M.; Pasta, S.Y.; Ramakrishna, T.; Naiki, H.; Goto, Y.; Rao Ch, M. AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. Biochem. J. 2005, 392, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Carroni, M.; Nussbaum-Krammer, C.; Mogk, A.; Nillegoda, N.B.; Szlachcic, A.; Guilbride, D.L.; Saibil, H.R.; Mayer, M.P.; Bukau, B. Human Hsp70 Disaggregase Reverses Parkinson’s-Linked α-Synuclein Amyloid Fibrils. Mol. Cell 2015, 59, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Binger, K.J.; Ecroyd, H.; Yang, S.; Carver, J.A.; Howlett, G.J.; Griffin, M.D.W. Avoiding the oligomeric state: αB-crystallin inhibits fragmentation and induces dissociation of apolipoprotein C-II amyloid fibrils. FASEB J. 2012, 27, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Waudby, C.A.; Knowles, T.P.; Devlin, G.L.; Skepper, J.N.; Ecroyd, H.; Carver, J.A.; Welland, M.E.; Christodoulou, J.; Dobson, C.M.; Meehan, S. The Interaction of αB-Crystallin with Mature α-Synuclein Amyloid Fibrils Inhibits Their Elongation. Biophys. J. 2010, 98, 843–851. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamashita, A.; Arakaki, N.; Nemoto, H.; Yamazaki, T. Prevention of aberrant protein aggregation by anchoring the molecular chaperone αB-crystallin to the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2014, 455, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Van De Schootbrugge, C.; Schults, E.M.; Bussink, J.; Span, P.N.; Grénman, R.; Pruijn, G.J.; Kaanders, J.H.; Boelens, W.C. Effect of hypoxia on the expression of αB-crystallin in head and neck squamous cell carcinoma. BMC Cancer 2014, 14, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D.; Carver, J.A.; Ecroyd, H. Preventing α-synuclein aggregation: The role of the small heat-shock molecular chaperone proteins. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1830–1843. [Google Scholar] [CrossRef] [Green Version]
- Rekas, A.; Adda, C.G.; Aquilina, J.A.; Barnham, K.J.; Sunde, M.; Galatis, D.; Williamson, N.A.; Masters, C.L.; Anders, R.F.; Robinson, C.V.; et al. Interaction of the Molecular Chaperone αB-Crystallin with α-Synuclein: Effects on Amyloid Fibril Formation and Chaperone Activity. J. Mol. Biol. 2004, 340, 1167–1183. [Google Scholar] [CrossRef]
- Milani, P.; Merlini, G.; Palladini, G. Light chain amyloidosis. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018022. [Google Scholar] [CrossRef] [Green Version]
- Blancas-Mejia, L.M.; Misra, P.; Dick, C.J.; Cooper, S.A.; Redhage, K.R.; Bergman, M.R.; Jordan, T.L.; Maar, K.; Ramirez-Alvarado, M. Immunoglobulin light chain amyloid aggregation. Chem. Commun. 2018, 54, 10664–10674. [Google Scholar] [CrossRef]
- Pleyer, C.; Flesche, J.; Saeed, F. Lysozyme amyloidosis – a case report and review of the literature. Clin. Nephrol. Case Stud. 2015, 3, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Scarpioni, R.; Ricardi, M.; Albertazzi, V.; De Amicis, S.; Rastelli, F.; Zerbini, L. Dialysis-related amyloidosis: Challenges and solutions. Int. J. Nephrol. Renov. Dis. 2016, 9, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulatskaya, A.I.; Rodina, N.P.; Polyakov, D.; Sulatsky, M.I.; Artamonova, T.; Khodorkovskii, M.; Shavlovsky, M.M.; Kuznetsova, I.M.; Turoverov, K. Structural Features of Amyloid Fibrils Formed from the Full-Length and Truncated Forms of Beta-2-Microglobulin Probed by Fluorescent Dye Thioflavin, T. Int. J. Mol. Sci. 2018, 19, 2762. [Google Scholar] [CrossRef] [Green Version]
- Sulatskaya, A.I.; Rodina, N.P.; Kuznetsova, I.M.; Turoverov, K.K. Different conditions of fibrillogenesis cause polymorphysm of lysozyme amyloid fibrils. J. Mol. Struct. 2017, 1140, 52–58. [Google Scholar] [CrossRef]
- Augusteyn, R.C.; Ellerton, H.D.; Putilina, T.; Stevens, A. Specific dissociation of alpha B subunits from alpha-crystallin. Biochim. Biophys. Acta 1988, 957, 192–201. [Google Scholar] [CrossRef]
- Provencher, S.W.; Gloeckner, J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 1981, 20, 33–37. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Stepanenko, O.V.; Stepanenko, O.V.; Povarova, O.I.; Biktashev, A.G.; Verkhusha, V.V.; Shavlovsky, M.M.; Turoverov, K.K. The Place of Inactivated Actin and Its Kinetic Predecessor in Actin Folding−Unfolding†. Biochemistry 2002, 41, 13127–13132. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.N.; Nagineni, C.N.; Bhat, S.P. alpha A-crystallin is expressed in non-ocular tissues. J. Biol. Chem. 1992, 267, 23337–23341. [Google Scholar]
- Iwaki, T.; Wisniewski, T.; Iwaki, A.; Corbin, E.; Tomokane, N.; Tateishi, J.; Goldman, J.E. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am. J. Pathol. 1992, 140, 345–356. [Google Scholar]
- Renkawek, K.; De Jong, W.W.; Merck, K.B.; Frenken, C.W.G.M.; Van Workum, F.P.A.; Bosman, G.J.C.G.M. αB-Crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol. 1992, 83, 324–327. [Google Scholar] [CrossRef]
- Renkawek, K.; Voorter, C.E.; Bosman, G.J.; van Workum, F.P.; de Jong, W.W. Expression of alpha B-crystallin in Alzheimer’s disease. Acta Neuropathol. 1994, 87, 155–160. [Google Scholar] [CrossRef]
- Esposito, G.; Garvey, M.; Alverdi, V.; Pettirossi, F.; Corazza, A.; Fogolari, F.; Polano, M.; Mangione, P.P.; Giorgetti, S.; Stoppini, M.; et al. Monitoring the interaction between beta2-microglobulin and the molecular chaperone alphaB-crystallin by NMR and mass spectrometry: AlphaB-crystallin dissociates beta2-microglobulin oligomers. J. Biol. Chem. 2013, 288, 17844–17858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanova, O.M.; Galzitskaya, O.V. Structural and Functional Peculiarities of α-Crystallin. Biology 2020, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Nørholm, A.-B.; Hendus-Altenburger, R.; Pedersen, S.F.; Poulsen, F.M.; Kragelund, B.B. Temperature-dependent structural changes in intrinsically disordered proteins: Formation of á-helices or loss of polyproline II? Protein Sci. 2010, 19, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999, 309, 274–284. [Google Scholar]
- Sulatskaya, A.I.; Rodina, N.P.; Sulatsky, M.I.; Povarova, O.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Investigation of α-Synuclein Amyloid Fibrils Using the Fluorescent Probe Thioflavin, T. Int. J. Mol. Sci. 2018, 19, 2486. [Google Scholar] [CrossRef] [Green Version]
- Hawe, A.; Sutter, M.; Jiskoot, W. Extrinsic Fluorescent Dyes as Tools for Protein Characterization. Pharm. Res. 2008, 25, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, M.; Sörgjerd, K.; Hammarström, P. Detection and Characterization of Aggregates, Prefibrillar Amyloidogenic Oligomers, and Protofibrils Using Fluorescence Spectroscopy. Biophys. J. 2005, 88, 4200–4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznetsova, I.M.; Sulatskaya, A.I.; Povarova, O.I.; Turoverov, K.K. Reevaluation of ANS Binding to Human and Bovine Serum Albumins: Key Role of Equilibrium Microdialysis in Ligand – Receptor Binding Characterization. PLoS ONE 2012, 7, e40845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulatsky, M.I.; Sulatskaya, A.I.; Povarova, O.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Effect of the fluorescent probes ThT and ANS on the mature amyloid fibrils. Prion 2020, 14, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sulatsky, M.; Sulatskaya, A.; Stepanenko, O.V.; Povarova, O.; Kuznetsova, I.; Turoverov, K.K. Denaturant effect on amyloid fibrils: Declasterization, depolymerization, denaturation and reassembly. Int. J. Biol. Macromol. 2020, 150, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Gong, H.; Xiao, H.; Petersen, R.B.; Zheng, L.; Huang, K. Inhibiting toxic aggregation of amyloidogenic proteins: A therapeutic strategy for protein misfolding diseases. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2013, 1830, 4860–4871. [Google Scholar] [CrossRef]
- Chowdhury, A.; Choudhury, A.; Banerjee, V.; Banerjee, R.; Das, K.P. Spectroscopic studies of the unfolding of a multimeric protein α-crystallin. Biopolymers 2014, 101, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K. The Alkaline Diet: Is There Evidence That an Alkaline pH Diet Benefits Health? J. Environ. Public Heal. 2011, 2012, 1–7. [Google Scholar] [CrossRef]
- Marieb, E.N.; Hoehn, K. Human Anatomy & Physiology, 11th ed.; Pearson Education: London, UK, 2007; (various pagings). [Google Scholar]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap((R)) System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Xue, W.-F.; Hellewell, A.L.; Gosal, W.S.; Homans, S.W.; Hewitt, E.W.; Radford, S.E. Fibril Fragmentation Enhances Amyloid Cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.-F.; Hellewell, A.L.; Hewitt, E.W.; Radford, S.E. Fibril fragmentation in amyloid assembly and cytotoxicity. Prion 2010, 4, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bystrenova, E.; Bednarikova, Z.; Barbalinardo, M.; Albonetti, C.; Valle, F.; Gazova, Z. Amyloid fragments and their toxicity on neural cells. Regen. Biomater. 2019, 6, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Mechanisms of enzymatic degradation of amyloid Beta microfibrils generating nanofilaments and nanospheres related to cytotoxicity. Biochemistry 2010, 49, 3254–3260. [Google Scholar] [CrossRef] [Green Version]
- Datskevich, P.N.; Mymrikov, E.V.; Sluchanko, N.N.; Shemetov, A.A.; Sudnitsyna, M.V.; Gusev, N.B. Expression, purification and some properties of fluorescent chimeras of human small heat shock proteins. Protein Expr. Purif. 2012, 82, 45–54. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Bukach, O.V.; Seit-Nebi, A.; Gusev, N.B. The pivotal role of the β7 strand in the intersubunit contacts of different human small heat shock proteins. Cell Stress Chaperon 2009, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nat. Cell Biol. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Turoverov, K.K.; Verkhusha, V.V.; Shavlovsky, M.M.; Biktashev, A.G.; Povarova, O.I.; Kuznetsova, I.M. Kinetics of Actin Unfolding Induced by Guanidine Hydrochloride†. Biochemistry 2002, 41, 1014–1019. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Litvin, F.F. Photobiology and spectroscopic methods. In Handbook of general biophisics, High school: Moscow, 1964; Vol. 8, pp 88-91. In Handbook of General Biophisics; High School: Moscow, Russia, 1964; Volume 8, pp. 88–91. [Google Scholar]
- Turoverov, K.K.; Biktashev, A.G.; Dorofeiuk, A.V.; Kuznetsova, I.M. A complex of apparatus and programs for the measurement of spectral, polarization and kinetic characteristics of fluorescence in solution. Tsitologiya 1998, 40, 8. [Google Scholar]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of Dyes in Solutions with High Absorbance. Inner Filter Effect Correction. PLoS ONE 2014, 9, e103878. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nishimura, S.; Murasugi, T.; Kubo, T.; Kaneko, I.; Meguro, M.; Marumoto, S.; Kogen, H.; Koyama, K.; Oda, T.; Nakagami, Y. RS-4252 inhibits amyloid beta-induced cytotoxicity in HeLa cells. Pharmacol. Toxicol. 2003, 93, 29–32. [Google Scholar] [CrossRef] [PubMed]







| Time of Fibrils Incubation in the Presence of αBCry | Lysozyme Fibrils (Prepared at pH 7) | Beta-2-Microglobulin Fibrils (Prepared at pH 2) | Lysozyme Fibrils (Prepared at pH 2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α, % | β, % | T, % | U,% | α, % | β, % | T, % | U, % | α, % | β, % | T, % | U, % | |
| 0 min | 2.6 | 48.3 | 18.1 | 31.0 | 7.3 | 43.9 | 26.1 | 22.7 | 14.8 | 32.0 | 23.1 | 30.1 |
| 20 min | 2.9 | 39.6 | 18.8 | 38.7 | 6.2 | 38.4 | 26.3 | 29.1 | 11.9 | 32.4 | 21.5 | 34.2 |
| 4 h | 2.7 | 38.4 | 18.1 | 40.8 | 6.7 | 38.5 | 22.5 | 32.3 | 8.0 | 32.9 | 22.2 | 36.9 |
| 1 day | 2.7 | 38.3 | 18.0 | 41.0 | 5.5 | 38.6 | 21.0 | 34.9 | 7.3 | 31.0 | 22.1 | 39.6 |
| 5–8 days | 2.6 | 38.0 | 18.6 | 40.8 | 4.5 | 38.5 | 21.3 | 35.7 | 4.9 | 32.0 | 21.8 | 41.3 |
| Characteristic | Lysozyme Fibrils (Prepared at pH 7) | Beta-2-Microglobulin Fibrils (Prepared at pH 2) | Lysozyme Fibrils (Prepared at pH 2) |
|---|---|---|---|
| RLS | 370 ± 7 | 204 ± 19 | 159 ± 15 |
| r | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 |
| A | 1.28 ± 0.04 | 1.47 ± 0.04 | 1.27 ± 0.04 |
| Ftotal·10−3 | 24.9 ± 0.8 | 10.4 ± 0.3 | 32.5 ± 1.0 |
| Ftotal_ThT·10−3 | 53 ± 1 | 21 ± 1 | 14 ± 1 |
| Ftotal_ANS·10−3 | 72.0 ± 2.8 | 8.7 ± 0.3 | 26.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, O.V.; Sulatsky, M.I.; Mikhailova, E.V.; Stepanenko, O.V.; Povarova, O.I.; Kuznetsova, I.M.; Turoverov, K.K.; Sulatskaya, A.I. Alpha-B-Crystallin Effect on Mature Amyloid Fibrils: Different Degradation Mechanisms and Changes in Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 7659. https://doi.org/10.3390/ijms21207659
Stepanenko OV, Sulatsky MI, Mikhailova EV, Stepanenko OV, Povarova OI, Kuznetsova IM, Turoverov KK, Sulatskaya AI. Alpha-B-Crystallin Effect on Mature Amyloid Fibrils: Different Degradation Mechanisms and Changes in Cytotoxicity. International Journal of Molecular Sciences. 2020; 21(20):7659. https://doi.org/10.3390/ijms21207659
Chicago/Turabian StyleStepanenko, Olga V., M. I. Sulatsky, E. V. Mikhailova, Olesya V. Stepanenko, O. I. Povarova, I. M. Kuznetsova, K. K. Turoverov, and A. I. Sulatskaya. 2020. "Alpha-B-Crystallin Effect on Mature Amyloid Fibrils: Different Degradation Mechanisms and Changes in Cytotoxicity" International Journal of Molecular Sciences 21, no. 20: 7659. https://doi.org/10.3390/ijms21207659