Evaluation of the Farming Potential of Echinacea Angustifolia DC. Accessions Grown in Italy by Root-Marker Compound Content and Morphological Trait Analyses
Abstract
:1. Introduction
2. Results
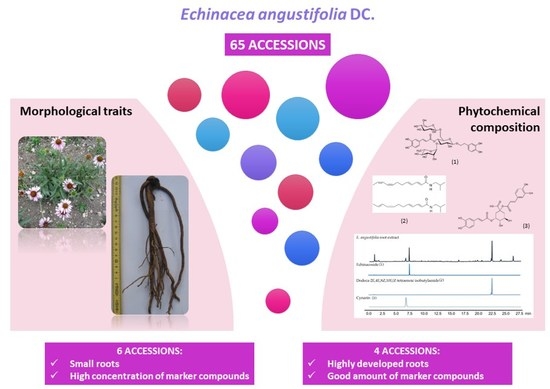
2.1. Marker-Compound Quantification and Morphological Confirmation of the Accessions
2.2. Phytochemical Evaluation and Morpho-Quantitative Characteristics of E. Angustifolia Accessions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Morpho-Quantitative Evaluation
4.3. Extraction of Plant Material
4.4. HPLC-PDA-MS/MS Analysis and Quantification
4.4.1. Qualitative Analysis
4.4.2. Quantitative Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, P.N.; Chan, M.; Betz, J.M. Optimization and single-laboratory validation study of a high-performance liquid chromatography (HPLC) method for the determination of phenolic Echinacea constituents. Anal. Bioanal. Chem. 2010, 397, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Mistríková, I.; Vaverková, Š. Morphology and anatomy of Echinacea purpurea, E. angustifolia, E. pallida and Parthenium integrifolium. Biologia 2007, 62, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Raclariu, A.C.; Ţebrencu, C.E.; Ichim, M.C.; Ciupercǎ, O.T.; Brysting, A.K.; de Boer, H. What’s in the box? Authentication of Echinacea herbal products using DNA metabarcoding and HPTLC. Phytomedicine 2018, 44, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Binns, S.E.; Baum, B.R.; Arnason, J.T. A taxonomic revision of echinacea (Asteraceae: Heliantheae). Syst. Bot. 2002, 27, 610–632. [Google Scholar]
- Mckeown, K.A. A Review of the Taxonomy of the Genus Echinacea; ASHS Press: Alexandria, VA, USA, 1999; pp. 482–498. [Google Scholar]
- Dall’Acqua, S.; Grabnar, I.; Verardo, R.; Klaric, E.; Marchionni, L.; Luidy-Imada, E.; Sut, S.; Agostinis, C.; Bulla, R.; Perissutti, B.; et al. Combined extracts of Echinacea angustifolia DC. and Zingiber officinale Roscoe in softgel capsules: Pharmacokinetics and immunomodulatory effects assessed by gene expression profiling. Phytomedicine 2019, 65, 153090. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species ( Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef] [Green Version]
- Aiello, N.; Carlini, A.; Scartezzini, F.; Fusani, P.; Berto, C.; Dall’Acqua, S. Harvest in different years of growth influences chemical composition of Echinacea angustifolia roots. Ind. Crops Prod. 2015, 76, 1164–1168. [Google Scholar] [CrossRef]
- Dapas, B.; Dall’Acqua, S.; Bulla, R.; Agostinis, C.; Perissutti, B.; Invernizzi, S.; Grassi, G.; Voinovich, D. Immunomodulation mediated by a herbal syrup containing a standardized Echinacea root extract: A pilot study in healthy human subjects on cytokine gene expression. Phytomedicine 2014, 21, 1406–1410. [Google Scholar] [CrossRef]
- Starvaggi Cucuzza, L.; Motta, M.; Accornero, P.; Baratta, M. Effect of Echinacea augustifolia extract on cell viability and differentiation in mammary epithelial cells. Phytomedicine 2008, 15, 555–562. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M.; Lasseigne, T. Variability in the composition of anti-oxidant compounds in Echinacea species by HPLC. Phytochem. Anal. 2005, 16, 77–85. [Google Scholar] [CrossRef]
- Willick, I.R.; Barl, B.; Tanino, K.K. Effect of latitude on narrow-leaved purple coneflower ( Echinacea angustifolia DC.) yield and phytochemical quality. Can. J. Plant. Sci. 2020, 100, 103–112. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Narrow-Leaved Coneflower Echinaceae Angustifoliae Radix, 10th ed.; European Pharmacopoeia: Strasbourg, France, 2020; pp. 1548–1549. [Google Scholar]
- Lucchesini, M.; Pacifici, S.; Maggini, R.; Pardossi, A.; Mensuali Sodi, A. A novel microfloating culture system for the in vitro rooting of Echinacea angustifolia D.C.: Photosynthetic performance and production of caffeic acid derivatives. Plant. Cell. Tissue Organ. Cult. 2019, 136, 123–132. [Google Scholar] [CrossRef]
- Lucchesini, M.; Bertoli, A.; Mensuali-Sodi, A.; Pistelli, L. Establishment of in vitro tissue cultures from Echinacea angustifolia D.C. adult plants for the production of phytochemical compounds. Sci. Hortic. 2009, 122, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Aiello, N.; Carlini, A.; Scartezzini, F.; Fusani, P.; Berto, C.; Dall’Acqua, S. Effect of growth substrates on morpho-quantitative and qualitative characteristics of Echinacea angustifolia var. angustifolia roots. J. Herbs Spices Med. Plants 2018, 24, 64–73. [Google Scholar] [CrossRef]
- Galambosi, B. Cultivation in Europe. In The Genus Echinacea; CRC PRESS: Boca Raton, FL, USA, 2004; pp. 41–64. [Google Scholar]
- Dall’Acqua, S.; Nicola, A.; Fabrizio, S.; Valentina, A.; Gabbriella, I. Analysis of highly secondary-metabolite producing roots and flowers of two Echinacea angustifolia DC. var. angustifolia accessions. Ind. Crops Prod. 2010, 31, 466–468. [Google Scholar]
- Binns, S.E.; Arnason, J.T.; Baum, B.R. Phytochemical variation within populations of Echinacea angustifolia (Asteraceae). Biochem. Syst. Ecol. 2002, 30, 837–854. [Google Scholar] [CrossRef]
- Wu, L.; Dixon, P.; Nikolau, B.; Kraus, G.; Widrlechner, M.; Wurtele, E. Metabolic profiling of Echinacea genotypes and a test of alternative taxonomic treatments. Planta Med. 2009, 75, 178–183. [Google Scholar] [CrossRef] [Green Version]
- McGregor, R.L. The taxonomy of the genus Echinacea (Compositae). Univ. Kansas Sci. Bull. 1968, 48, 113–142. [Google Scholar]
- Chuanren, D.; Bochu, W.; Wanqian, L.; Jing, C.; Jie, L.; Huan, Z. Effect of chemical and physical factors to improve the germination rate of Echinacea angustifolia seeds. Coll. Surf. B Biointerf. 2004, 37, 101–105. [Google Scholar] [CrossRef]
- Pietta, P.; Mauri, P.; Fuzzati, N. Analytical Profiles of Echinacea Species. In The Genus Echinacea; CRC PRESS: Boca Raton, FL, USA, 2004; pp. 101–117. [Google Scholar]
- Greenfield, J.; Davis, J. Echinacea (Echinacea Angustifolia) 2012. Available online: https://newcropsorganics.ces.ncsu.edu/wp-content/uploads/2017/06/Echinacea-Angustifolia.pdf (accessed on 8 July 2020).
- Jedrzejczyk, I. Genome size and SCoT markers as tools for identification and genetic diversity assessment in Echinacea genus. Ind. Crops Prod. 2020, 144, 112055. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Hevia, F. Effect of harvest season, nitrogen, phosphorus and potassium on root yield, echinacoside and alkylamides in Echinacea angustifolia L. in Chile. Acta Hortic. 2002, 576, 303–310. [Google Scholar] [CrossRef]
- Kabganian, R.; Carrier, D.J.; Sokansanj, S. Drying of Echinacea angustifolia roots. J. Herbs Spices Med. Plants 2008, 10, 11–18. [Google Scholar] [CrossRef]





| Accession N° | State | PI Accession/Other Origin |
|---|---|---|
| 1 | Nebraska, USA | Ames 03075 |
| 2 | USA | Ames 31323 |
| 3 | Nebraska, USA | PI 312814 |
| 4 | Oklahoma, USA | PI 421332 |
| 5 | Kansas | PI 597601 |
| 6 | Oklahoma | PI 631267 |
| 7 | Minnesota | PI 649026 |
| 8 | Maine | PI 664823 |
| 9 | Nebraska | PI 421331 |
| 10 | Nebraska | PI 421372 |
| 11 | Oklahoma | PI 631261 |
| 12 | Oklahoma | PI 631268 |
| 13 | Oklahoma | PI 631269 |
| 14 | Oklahoma | PI 631270 |
| 15 | Oklahoma | PI 631271 |
| 16 | Oklahoma | PI 631272 |
| 17 | Oklahoma | PI 631273 |
| 18 | Iowa | PI 631283 |
| 19 | Iowa | PI 631284 |
| 20 | Iowa | PI 631285 |
| 21 | Iowa | PI 631286 |
| 22 | Iowa | PI 631287 |
| 23 | Iowa | PI 631288 |
| 24 | Iowa | PI 631289 |
| 25 | Kansas | PI 631317 |
| 26 | Kansas | PI 631318 |
| 27 | Kansas | PI 631319 |
| 28 | Iowa | PI 633650 |
| 29 | Iowa | PI 633651 |
| 30 | North Dakota | PI 633652 |
| 31 | North Dakota | PI 633653 |
| 32 | North Dakota | PI 633654 |
| 33 | North Dakota | PI 633655 |
| 34 | North Dakota | PI 633656 |
| 35 | North Dakota | PI 633657 |
| 36 | Montana | PI 636393 |
| 37 | North Dakota | PI 636394 |
| 38 | North Dakota | PI 636395 |
| 39 | North Dakota | PI 636396 |
| 40 | Iowa | PI 649027 |
| 41 | Iowa | PI 649028 |
| 42 | North Dakota | PI 649029 |
| 43 | North Dakota | PI 649030 |
| 44 | North Dakota | PI 649031 |
| 45 | Oklahoma | PI 649032 |
| 46 | South Dakota | PI 649033 |
| 47 | North Dakota | PI 664824 |
| 48 | Wyoming | PI 664825 |
| 49 | Wyoming | PI 631266 |
| 50 | Oklahoma, USA | PI 631320 |
| 51 | Goodwood, ON, Canada | Richters Herbs |
| 52 | Oregon, USA | Horizon Herbs |
| 53 | Arnhem, Netherlands | Open Lucht Museum |
| 54 | Ulm, Germany | Botanic Garden |
| 55 | Bonn, Germany | Botanic Garden |
| 56 | Bonn, Germany | Botanic Garden |
| 57 | Talence, France | Botanic Garden |
| 58 | Italy | Veneto Farm |
| 59 | Trento, Italy | CREA-FL |
| 60 | Trento, Italy | CREA-FL |
| 61 | Trento, Italy | CREA-FL |
| 62 | Trento, Italy | CREA-FL |
| 63 | Cultus Lake, BC, Canada | Strobl Farm |
| 64 | Cultus Lake, BC, Canada | Strobl Farm |
| 65 | Cultus Lake, BC, Canada | Strobl Farm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, N.; Marengo, A.; Scartezzini, F.; Fusani, P.; Sgorbini, B.; Rubiolo, P.; Cagliero, C. Evaluation of the Farming Potential of Echinacea Angustifolia DC. Accessions Grown in Italy by Root-Marker Compound Content and Morphological Trait Analyses. Plants 2020, 9, 873. https://doi.org/10.3390/plants9070873
Aiello N, Marengo A, Scartezzini F, Fusani P, Sgorbini B, Rubiolo P, Cagliero C. Evaluation of the Farming Potential of Echinacea Angustifolia DC. Accessions Grown in Italy by Root-Marker Compound Content and Morphological Trait Analyses. Plants. 2020; 9(7):873. https://doi.org/10.3390/plants9070873
Chicago/Turabian StyleAiello, Nicola, Arianna Marengo, Fabrizio Scartezzini, Pietro Fusani, Barbara Sgorbini, Patrizia Rubiolo, and Cecilia Cagliero. 2020. "Evaluation of the Farming Potential of Echinacea Angustifolia DC. Accessions Grown in Italy by Root-Marker Compound Content and Morphological Trait Analyses" Plants 9, no. 7: 873. https://doi.org/10.3390/plants9070873