The Entrapment of Somatostatin in a Lipid Formulation: Retarded Release and Free Radical Reactivity
Abstract
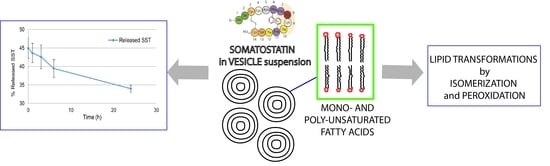
:1. Introduction
2. Results and Discussion
2.1. Release Experiments in Aqueous Citrate Buffer
2.2. Release Experiments in Human Plasma
2.3. Reactivity of SST in Liposome Vesicles
3. Conclusions
4. Materials and Methods
4.1. Preparation of Nanoemulsions (NE)
4.2. LC/MS Analysis
4.3. In Vitro Release Experiments in Buffer
4.4. In Vitro Release Profile in Human Plasma
4.5. Liposome Experiments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weckbecker, G.; Raulf, F.; Stolz, B.; Bruns, C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol. Ther. 1994, 60, 245–264. [Google Scholar] [CrossRef]
- Raulf, F.; Pérez, J.; Hoyer, D.; Bruns, C. Differential expression of five somatostatin receptor subtypes, SSTR1-5, in the CNS and peripheral tissue. Digestion 1994, 55 (Suppl. 3), 46–53. [Google Scholar] [CrossRef]
- Lamberts, S.W.; Krenning, E.P.; Reubi, J.C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr. Rev. 1991, 12, 450–482. [Google Scholar] [CrossRef] [PubMed]
- Scarpignato, C.; Pelosini, L. Somatostatin analogs for cancer treatment and diagnosis: An overview. Chemotherap 2001, 47 (Suppl. 2), 1–29. [Google Scholar] [CrossRef]
- Florio, T.; Morini, M.; Villa, V.; Arena, S.; Corsaro, A.; Thellung, S.; Culler, M.D.; Pfeffer, U.; Noonan, D.M.; Schettini, G.; et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology 2003, 144, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, H.; Guillermet, J.; Hortala, M.; Vernejoul, F.; Pyronnet, S.; Bousquet, C.; Susini, C. Molecular signaling of somatostatin receptors. Ann. N. Y. Acad. Sci. 2004, 1014, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Lucius, R.; Mentlein, R. Degradation of the neuropeptide somatostatin by cultivated neuronal and glial cells. J. Biol. Chem. 1991, 266, 18907–18913. [Google Scholar] [PubMed]
- Biron, E.; Chatteriee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H.A.; Jelinek, R.; Gilon, C.; Hoffman, A.; et al. Improving oral bioavailability of peptides by multiple N-methylation: Somatostatin analogues. Angew. Chem. Int. Ed. 2008, 27, 2505–2599. [Google Scholar] [CrossRef]
- Demgé, C.; Vonderscher, J.; Marbach, P.; Pinget, M. Poly(alkyl cyanoacrylate) nanocapsules as delivery system in the rat for octreotide, a long-acting somatostatin analogue. J. Pharm. Pharm. 1997, 49, 949–954. [Google Scholar] [CrossRef]
- Sanarova, E.; Lantsova, A.; Oborotova, N.; Polozkova, A.; Dmitrieva, M.; Orlova, O.; Nikolaeva, L.; Borisova, L.; Shprakh, Z. Development of a liposomal dosage form for a new somatostatin analogue. Indian J. Pharm. Sci. 2019, 81, 146–149. [Google Scholar] [CrossRef]
- Helbok, A.; Rangger, C.; von Guggenberg, E.; Saba-Lepek, M.; Radolf, T.; Thurner, G.; Andreae, F.; Prassi, R.; Decristoforo, C. Targeting properties of peptide-modified radiolabeled liposomal nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Torchilin, V.P. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug Deliv. 2013, 2013, 705265. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.J.; Cheng, L.; Peng, X.M.; Wang, T.; Li, C.Q.; Song, X.L.; Liu, S.; Chao, J.P.; Li, X.T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 616–628. [Google Scholar] [CrossRef] [Green Version]
- Chatzisideri, T.; Leonidis, G.; Sarli, V. Cancer-targeted delivery systems based on peptides. Future Med. Chem. 2018, 10, 2201–2226. [Google Scholar] [CrossRef] [PubMed]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Giacometti, G.; Marini, M.; Papadopoulos, K.; Ferreri, C.; Chatgilialoglu, C. Trans-double bond-containing liposomes as potential carriers for drug delivery. Molecules 2017, 22, 2082. [Google Scholar] [CrossRef]
- Ferreri, C.; Pierotti, S.; Barbieri, A.; Zambonin, L.; Landi, L.; Rasl, S.; Luisi, P.L.; Barigelletti, F.; Chatgilialoglu, C. Comparison of phosphatidylcholine vesicle properties related to geometrical isomerism. Photochem. Photobiol. 2006, 82, 274–280. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Sansone, A.; Barata-Vallejo, S.; Chatgilialoglu, C.; Ferreri, C. Bleomycin-induced trans lipid formation in cell membranes and in liposome models. Org. Biomol. Chem. 2015, 13, 1100–1105. [Google Scholar] [CrossRef]
- Isoelectric Point. Available online: https://www.mybiosource.com/sst-recombinant-protein/somatostatin-sst/2011722 (accessed on 24 August 2019).
- Reithmeier, H.; Herrmann, J.; Göpferich, A. Development and characterization of lipid microparticles as a drug carrier for somatostatin. Int. J. Pharm. 2001, 218, 133–143. [Google Scholar] [CrossRef]
- Sakurai, H.; Yokoyama, A.; Tanaka, H. Studies on the sulfur-containing chelating agents. XXXI. Catalytic effect of copper (II) ion to formation of mixed disulfide. Chem. Pharm. Bull. 1971, 19, 1416–1423. [Google Scholar] [CrossRef]
- Antholine, W.E.; Petering, D.H. On the reaction of iron bleomycin with thiols and oxygen. Biochem. Biophys. Res. Commun. 1979, 90, 384–389. [Google Scholar] [CrossRef]
- Anoop, A.; Ranganathan, S.; Das Dhaked, B.; Nath Jha, N.; Pratihal, S.; Ghosh, S.; Sahav, S.; Kumar, S.; Das, S.; Kombrabail, M.; et al. Elucidating the role of disulfide bond on amyloid formation and fibril reversibility of somatostatin-14. J. Biol. Chem. 2014, 289, 16884–16903. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Sassatelli, F.; Samadi, A.; Landi, L.; Chatgilialoglu, C. Regioselective cis−trans isomerization of arachidonic double bonds by thiyl radicals: the influence of phospholipid supramolecular organization. J. Am. Chem. Soc. 2004, 126, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 36, 441–448. [Google Scholar]
- Chatgilialoglu, C.; Ferreri, C.; Guerra, M.; Samadi, A.; Bowry, V. The reaction of thiyl radical with methyl linoleate: Completing the picture. J. Am. Chem. Soc. 2017, 139, 4704–4714. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Herreruela, D.; Monnappa, A.K.; Muñoz-Úbeda, M.; Morallón-Piñ, A.; Enciso, E.; Sánchez, E.; Giusti, F.; Natale, P.; López-Montero, I. Lipid–peptide bioconjugation through pyridyl disulfide reaction chemistry and its application in cell targeting and drug delivery. J. Nanobiotechnol. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Yokohama, C.; Sueyoshi, Y.; Ema, M.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [Green Version]
- Toniolo, G.; Louka, M.; Menounou, G.; Fantoni, N.Z.; Mitrikas, G.; Efthimiadou, E.K.; Masi, A.; Bortolotti, M.; Polito, L.; Bolognesi, A.; et al. [Cu(TPMA)(Phen)](ClO4)2: Metallodrug nanocontainer delivery and membrane lipidomics of a neuroblastoma cell line coupled with a liposome biomimetic model focusing on fatty acid reactivity. ACS Omega 2018, 3, 15952–15965. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds “nanoemulsions of SST” are available from the authors. |






| Entry | Liposome | O2 | Peptide | Fe(II) | Fe(III) | trans-18:1 (%) | trans-18:2 (%) | PUFA Consumption (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | POPC 2 | no | SST | x | tr | |||
| 2 | POPC 2 | no | SST | x | 0.9 ± 0.1 | |||
| 3 | POPC 2 | no | red-SST | x | 0.7 ± 0.2 | |||
| 4 | POPC 2 | no | red-SST | x | 29.9 ± 0.2 | |||
| 5 | POPC 2 | yes | red-SST | x | 5 ± 0.3 | |||
| 6 | soybean lecithin 3 | yes | SST | nd | nd | 2.2 ± 1.4 | ||
| 7 | soybean lecithin 3 | yes | SST | x | tr | tr | 95.9 ± 0.3 | |
| 8 | soybean lecithin 3 | yes | SST | x4 | 0.3 ± 0.0 | 0.2 ± 0.1 | 75.5 ± 0.4 | |
| 9 | soybean lecithin 3 | yes | red-SST | x4 | 0.4 ± 0.1 | 0.5 ± 0.1 | 84.1 ± 2.6 | |
| 10 | soybean lecithin 3 | yes | SST | x4 | tr | tr | 35.9± 0.2 | |
| 11 | soybean lecithin 3 | yes | red-SST | x4 | tr | tr | 92.3± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larocca, A.V.; Toniolo, G.; Tortorella, S.; Krokidis, M.G.; Menounou, G.; Di Bella, G.; Chatgilialoglu, C.; Ferreri, C. The Entrapment of Somatostatin in a Lipid Formulation: Retarded Release and Free Radical Reactivity. Molecules 2019, 24, 3085. https://doi.org/10.3390/molecules24173085
Larocca AV, Toniolo G, Tortorella S, Krokidis MG, Menounou G, Di Bella G, Chatgilialoglu C, Ferreri C. The Entrapment of Somatostatin in a Lipid Formulation: Retarded Release and Free Radical Reactivity. Molecules. 2019; 24(17):3085. https://doi.org/10.3390/molecules24173085
Chicago/Turabian StyleLarocca, Anna Vita, Gianluca Toniolo, Silvia Tortorella, Marios G. Krokidis, Georgia Menounou, Giuseppe Di Bella, Chryssostomos Chatgilialoglu, and Carla Ferreri. 2019. "The Entrapment of Somatostatin in a Lipid Formulation: Retarded Release and Free Radical Reactivity" Molecules 24, no. 17: 3085. https://doi.org/10.3390/molecules24173085