Design, Synthesis, and SAR of Novel 2-Glycinamide Cyclohexyl Sulfonamide Derivatives against Botrytis cinerea
Abstract
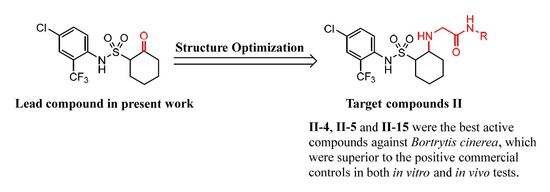
:1. Introduction
2. Result and Discussion
2.1. Chemistry
2.2. Biological Activity and Structure–Activity Relationship Study
3. Materials and Method
3.1. Materials and Instrumentation
3.2. Synthesis
3.2.1. Synthesis of N-(2-Trifluoromethyl-4-chlorophenyl)-2-aminocyclohexyl Sulfonamide L-2
3.2.2. Synthesis of Substituted Chloroacetamide I
3.2.3. Synthesis of 2-Glycinamide Cyclohexyl Sulfonamide Derivatives II-1 to II-28
3.2.4. Synthesis of N-(2-Trifluoromethyl-4-chlorophenyl)-2-ethoxyacylmethyl Amino Cyclohexyl Sulfonamide L-4 and N-(2-Trifluoromethyl-4-chlorophenyl)-2-carboxymethyl Amino Cyclohexyl Sulfonamide L-5
3.2.5. Synthesis of 2-Glycinamide Cyclohexyl Sulfonamide Derivatives II-29 to II-33
3.3. Fungal Bioassay
3.3.1. Evaluation of Target Compounds II on the Inhibition of B. cinerea
3.3.2. Evaluation of the Fungicidal Activity on B. cinerea by Concentration Gradient Test
3.3.3. In Vivo Fungicidal Activity against B. cinerea (CY-09) by Greenhouse Pot Experiments
(1). Evaluation of Fungicidal Activity on Cucumber Leaves
(2). Evaluation of Fungicidal Activity on Tomato Leaves
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.R.; Pan, H.Q.; et al. The genome sequence of the rice blast fungus magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Gurr, S.; Samalova, M.; Fisher, M. The rise and rise of emerging infectious fungi challenges food security and ecosystem health. Fungal Biol. Rev. 2011, 25, 181–188. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Fu, H.; Zhou, Q.; Strelkov, S.E.; Turnbull, G.D. First report of Phytophthora sansomeana causing root rot in field pea in Alberta, Canada. Crop. Prot. 2017, 101, 1–4. [Google Scholar] [CrossRef]
- Gladieux, P.; Byrnes, E.J.; Aguileta, G.; Fisher, M.; Billmyre, R.B.; Heitman, J.; Giraud, T. Epidemiology and evolution of fungal pathogens in plants and animals. Genet. Evol. Infect. Dis. 2017, 71–98. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Kan, J.A.L.V. Botrytis cinerea: The cause of grey mould disease. Mol. Plant. Pathol. 2017, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple resistance of botrytis cinerea from kiwifruit to sdhis, qois and fungicides of other chemical groups. Pest. Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Che, Z.; Chen, G. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop. Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Panebianco, A.; Castello, I.; Cirvilleri, G.; Perrone, G.; Epifani, F.; Ferrara, M.; Polizzi, G.; Walters, D.R.; Vitale, A. Detection of Botrytis cinerea field isolates with multiple fungicide resistance from table grape in Sicily. Crop. Prot. 2015, 77, 65–73. [Google Scholar] [CrossRef]
- Rm, D.M.A.; Rotolo, C.; Masiello, M.; Gerin, D.; Pollastro, S.; Faretra, F. Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest. Manag. Sci. 2014, 70, 1785–1796. [Google Scholar]
- Khalaf, A.I.; Anthony, N.; Breen, D.; Donoghue, G.; Mackay, S.P.; Scott, F.J.; Suckling, C.J. Amide isosteres in structure-activity studies of antibacterial minor groove binders. Eur. J. Med. Chem. 2011, 46, 5343–5355. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Xie, D.; Yin, L.; Wang, Z.; Chen, J.; Zhang, A.; Song, B.; Hu, D. Novel alpha, β-unsaturated amide derivatives bearing α-amino phosphonate moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2017, 27, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Singh, J.; Kumar, A.; Jamal, H.; Gupta, P.P. Synthesis of amidine and amide derivatives and their evaluation for anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.X.; Chen, L.L.; Li, H.B.; Liu, F.J.; Hu, J.Q.; Hu, W.X. Synthesis and biological evaluation of amide derivatives of diflunisal as potential anti-tumor agents. Bioorg. Med. Chem. Lett. 2009, 19, 4399–4402. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J.; Alam, M.J.; Nawaz, F.; Naidu, V.G.M.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, molecular docking and anti-diabetic evaluation of 2,4-thiazolidinedione based amide derivatives. Bioorg. Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rui, P.; Liu, C.; Du, Y.; Qin, P.; Qi, Z.; Ji, M.; Li, X.; Cui, Z. Design, synthesis and fungicidal activity of 2-substituted phenyl-2-oxo-, 2-hydroxy- and 2-acyloxyethylsulfonamides. Molecules 2017, 22, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Zhang, J.J.; Yan, X.J.; Liang, X.M.; Wang, D.Q. A novel fungicide chesulfamide. Agrochemicals 2012, 51, 287–288. [Google Scholar]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.H.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenyl-sulfonamides and their activity against Botrytis cinerea. Pest. Manag. Sci. 2011, 67, 986–992. [Google Scholar] [CrossRef] [PubMed]
- China Agricultural University. Novel fungicide-Chesulfamide. World Pestic. 2012, 34, 56. [Google Scholar]
- Li, X.; Yang, X.; Liang, X.; Kai, Z.; Yuan, H.; Yuan, D.; Zhang, J.; Wang, R.; Ran, F.; Qi, S.; et al. Synthesis and biological activities of 2-oxocycloalkylsulfonamides. Bioorg. Med. Chem. 2008, 16, 4538–4544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Wu, D.C.; Qi, Z.Q.; Gu, Z.M.; Li, X.W.; Ji, M.S. Fungicidal activity of 2-oxo and 2-hydroxycycloalkylsulfonamides against 14 fungus species. Chin. J. Pestic. Sci. 2011, 13, 423–426. [Google Scholar]
- Li, X.; Cui, Z.; Chen, X.; Wu, D.; Qi, Z.; Ji, M. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int. J. Mol. Sci. 2013, 14, 22544–22557. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Wu, D.; Ji, M. Combinatorial synthesis and fungicidal activity of 2-acyloxycyclohexylsulfonamides. Chin. J. Pestic. Sci. 2014, 16, 651–658. [Google Scholar]
- Liu, C.; Yan, X.; Wang, M.; Qin, P.; Qi, Z.; Ji, M.; Liu, X.; Vijaya Babu, P.; Li, X.; Cui, Z. Design, synthesis and fungicidal activity of novel 2-substituted aminocycloalkylsulfonamides. Bioorg. Med. Chem. Lett. 2017, 27, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qu, S.; Ji, M.; Qi, Z.; Li, X. Synthesis and fungicidal activity of 2-pyridinecarboxamide cyclohexylsulfonamides. Chin. J. Pestic. Sci. 2017, 19, 169–175. [Google Scholar]
- Zhao, J.; Qu, W.; Lin, D.; Kong, L.; Huang, Q.; Li, F.; She, D. Application of α-amino acids in agrochemicals synthesis. Chin. J. Pestic. Sci. 2010, 12, 371–382. [Google Scholar]
- Jain, P.; Wadhwa, P.K.; Gunapati, S.; Jadhav, H.R. Design, synthesis and in vitro evaluation studies of sulfonyl-amino-acetamides as small molecule BACE-1 inhibitors. Bioorg. Med. Chem. 2016, 24, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.M.; Bursavich, M.G.; Lombardi, S.; Georgiadis, K.E.; Reifenberg, E.; Flannery, C.R.; Morris, E.A. N-((8-hydroxy-5-substituted-quinolin-7-yl)(phenyl)methyl)-2-phenyloxy/amino-aceta mide inhibitors of ADAMTS-5 (Aggrecanase-2). Bioorg. Med. Chem. Lett. 2008, 18, 6454–6457. [Google Scholar] [CrossRef] [PubMed]
- Pawar, C.D.; Sarkate, A.P.; Karnik, K.S.; Bahekar, S.S.; Pansare, D.N.; Shelke, R.N.; Jawale, C.S.; Shinde, D.B. Synthesis and antimicrobial evaluation of novel ethyl 2-(2-(4-substituted)acetamido)-4-subtituted-thiazole-5-carboxylate derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3525–3528. [Google Scholar] [CrossRef] [PubMed]
- Dockendorff, C.; Faloon, P.W.; Germain, A.; Yu, M.; Youngsaye, W.; Nag, P.P.; Bennion, M.; Penman, M.; Nieland, T.J.; Dandapani, S.; et al. Discovery of bisamide-heterocycles as inhibitors of scavenger receptor BI (SR-BI)-mediated lipid uptake. Bioorg. Med. Chem. Lett. 2015, 25, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Lee, H.G.; Kang, S.-B.; Sung, G.H.; Kim, J.-J.; Park, J.K.; Lee, S.-G.; Yoon, Y.-J. tert-butoxide-assisted amidation of esters under green conditions. Synthesis 2012, 44, 42–50. [Google Scholar]
- Mark, W.; Bundesmann, S.B.C.; Stephen, W. Wright, Amidation of esters assisted by Mg(OCH3)2 or CaCl2. Tetrahedron. Lett. 2010, 51, 3879–3882. [Google Scholar]
- Li, X.; Liu, C.; Ji, M.; Qi, Z.; Qin, P.; Zhang, Y. 2-Substituted Aminocycloalkylsulfonamide Compounds and Their Preparation and Application. Patent CN 106316897 A, 11 January 2017. [Google Scholar]
- Liu, C.; Chen, X.; Qin, P.; Qi, Z.; Ji, M.; Liu, X.; Vijaya Babu, P.; Li, X.; Cui, Z. Synthesis, fungicidal activity, and structure activity relationship of β-acylaminocycloalkylsulfonamides against Botrytis cinerea. Sci. Rep. 2017, 7, 42096. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, R.N.; Nagle, A.S.; Jung, K.W. Cheminform abstract: cesium effect: high chemoselectivity in direct N-alkylation of amines. J. Org. Chem. 2002, 67, 674–683. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17980.28-2000. Pesticide field efficacy test guidelines (1). In Fungicide Control Botrytis Cinerea, China; State Bureau of Quality and Technical Supervision: Shenzhen, China, 1 May 2000. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |






| Compd. | R | Inhibition Rate (%) | EC50 Values |
|---|---|---|---|
| II-1 | C6H5– | 12.49 | >100 |
| II-2 | 3-F–C6H4– | 51.08 | >100 |
| II-3 | 4-F–C6H4– | 76.89 | 16.23 |
| II-4 | 2,4-2F–C6H3– | 90.66 | 4.01 |
| II-5 | 2,5-2F–C6H3– | 85.74 | 3.38 |
| II-6 | 2-F–4-Cl–C6H3– | 89.18 | 3.38 |
| II-7 | 2-F–4-Br–C6H3– | 81.32 | 9.44 |
| II-8 | 3-F–4-Br–C6H3– | 84.76 | 7.23 |
| II-9 | 2-CF3–4-F–C6H3– | 84.51 | 23.57 |
| II-10 | 2-F–5-CF3–C6H3– | 69.27 | 13.66 |
| II-11 | 3-Br–4F–C6H3– | 72.71 | 12.68 |
| II-12 | 3-CN–4-F–C6H3– | 90.41 | 16.27 |
| II-13 | 2,3,4-3F–C6H2– | 90.90 | 14.67 |
| II-14 | 2,4,5-3F–C6H2– | 88.94 | 8.9 |
| II-15 | 2-Br–C6H4– | 85.25 | 4.99 |
| II-16 | 3-Br–C6H4– | 53.05 | >100 |
| II-17 | 2,4-2Br–C6H3– | 73.45 | 16.48 |
| II-18 | 2-CH3–C6H4– | 33.87 | >100 |
| II-19 | 2-CF3–C6H4– | 82.79 | 3.26 |
| II-20 | 3-CF3–C6H4– | 6.59 | >100 |
| II-21 | 3,5-2CF3–C6H3– | 31.91 | >100 |
| II-22 | 3-CH3O–C6H4– | 14.70 | >100 |
| II-23 | 4-CH3O–C6H4– | 33.38 | >100 |
| II-24 | 2-CF3O–C6H4– | 51.57 | >100 |
| II-25 | 2-Cl-C6H4-CH2– | 56.00 | 8.02 |
| II-26 | 2-Br–C6H4–CH2– | 88.20 | 3.4 |
| II-27 | 2-CH3–C6H4–CH2– | 66.81 | 15.13 |
| II-28 | 2-Br–C6H4–CH2CH2– | 75.42 | 3.57 |
| II-29 | CH3CH2– | 55.75 | 34.18 |
| II-30 | CH3(CH2)2– | 75.42 | 5.9 |
| II-31 |  | 69.52 | 3.77 |
| II-32 |  | 81.32 | 14.07 |
| II-33 | CH3(CH2)3– | 83.78 | 6.5 |
| Carbendazim | / | 33.87 | 867.82 |
| Procymidone | / | 69.27 | 10.31 |
| Boscalid | / | 89.18 | 4.46 |
| Compd. | R | HLD-15 | DL-11 | ||
|---|---|---|---|---|---|
| Inhibition Rate (%) | EC50 Values | Inhibition Rate (%) | EC50 Values | ||
| II-4 | 2,4-2F–C6H3– | 83.41 | 1.88 | 89.47 | 2.07 |
| II-5 | 2,5-2F–C6H3– | 83.93 | 2.3 | 87.63 | 2.55 |
| II-6 | 2-F–4-Cl–C6H3– | 71.75 | 2.13 | 91.57 | 3.94 |
| II-26 | 2-Br–C6H4–CH2– | 70.19 | 2.96 | 94.47 | 2.92 |
| II-27 | 2-CH3–C6H4–CH2– | 79.52 | 7.25 | 78.94 | 5.55 |
| II-28 | 2-Br–C6H4–CH2CH2– | 96.89 | 2.26 | 95.00 | 2.47 |
| II-30 | CH3(CH2)2– | 84.19 | 7.12 | 86.05 | 5.79 |
| II-31 |  | 75.12 | 5.31 | 65.24 | 4.36 |
| II-33 | CH3(CH2)3– | 76.67 | 6.34 | 95.26 | 2.97 |
| Carbendazim | / | 10.32 | 427.78 | 10.48 | 673.38 |
| Procymidone | / | 79.00 | 10.13 | 75.25 | 8.61 |
| Boscalid | / | 87.82 | 2.44 | 86.57 | 2.27 |
| Compd. | Inhibition Rate (%) | Compd. | Inhibition Rate (%) |
|---|---|---|---|
| II-2 | 33.33 | II-17 | 59.52 |
| II-3 | 79.76 | II-18 | 20.83 |
| II-4 | 90.48 | II-20 | 64.88 |
| II-5 | 93.45 | II-23 | 27.38 |
| II-6 | 86.31 | II-24 | 71.43 |
| II-7 | 80.36 | II-25 | 35.71 |
| II-8 | 79.17 | II-26 | 53.57 |
| II-9 | 73.81 | II-27 | 37.50 |
| II-10 | 72.62 | II-28 | 51.19 |
| II-11 | 62.50 | II-29 | 66.07 |
| II-12 | 92.86 | II-30 | 42.86 |
| II-13 | 91.07 | II-31 | 63.09 |
| II-14 | 75.60 | II-32 | 71.43 |
| II-15 | 88.69 | II-33 | 75.00 |
| II-16 | 30.95 | Pyrimethanil | 82.14 |
| Carbendazim | 59.52 | Boscalid | 88.10 |
| Procymidone | 83.33 | Cyprodinil | 88.69 |
| Compd. | 500 μg mL−1 | 200 μg mL−1 | ||
|---|---|---|---|---|
| Disease Index | Control Efficacy (%) | Disease Index | Control Efficacy (%) | |
| II-2 | 5.82 | 74.43 | 11.37 | 70.98 |
| II-4 | 7.47 | 67.19 | 8.42 | 78.51 |
| II-5 | 7.19 | 68.43 | 8.26 | 78.91 |
| II-6 | 18.66 | 18.04 | / | / |
| II-7 | 5.96 | 73.83 | 12.10 | 69.11 |
| II-8 | 4.52 | 80.14 | 4.71 | 87.98 |
| II-10 | 12.69 | 44.27 | / | / |
| II-11 | 11.28 | 50.46 | / | / |
| II-13 | 21.39 | 6.04 | / | / |
| II-14 | 9.73 | 57.27 | / | / |
| II-15 | 5.25 | 76.96 | 4.71 | 87.97 |
| II-16 | 19.71 | 13.43 | / | / |
| II-17 | 14.52 | 36.25 | / | / |
| II-18 | 14.72 | 35.33 | / | / |
| II-19 | 17.21 | 24.40 | / | / |
| II-20 | 21.71 | 4.66 | / | / |
| II-24 | 20.99 | 7.81 | / | / |
| II-26 | 13.30 | 41.57 | / | / |
| II-28 | 8.21 | 63.93 | 12.61 | 67.83 |
| II-29 | 9.17 | 59.74 | / | / |
| II-30 | 11.56 | 49.21 | / | / |
| II-31 | 8.58 | 62.33 | 15.08 | 61.51 |
| II-32 | 16.02 | 29.63 | / | / |
| II-33 | 6.37 | 72.02 | 9.74 | 75.15 |
| Pyrimethanil | 15.25 | 33.00 | 19.62 | 49.93 |
| Procymidone | 5.91 | 74.05 | 13.45 | 65.67 |
| Boscalid | 5.23 | 77.05 | 8.58 | 78.10 |
| CK | 22.77 | / | 39.18 | / |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, N.; Liu, C.; Feng, Z.; Li, X.; Qi, Z.; Ji, M.; Qin, P.; Ahmed, W.; Cui, Z. Design, Synthesis, and SAR of Novel 2-Glycinamide Cyclohexyl Sulfonamide Derivatives against Botrytis cinerea. Molecules 2018, 23, 740. https://doi.org/10.3390/molecules23040740
Cai N, Liu C, Feng Z, Li X, Qi Z, Ji M, Qin P, Ahmed W, Cui Z. Design, Synthesis, and SAR of Novel 2-Glycinamide Cyclohexyl Sulfonamide Derivatives against Botrytis cinerea. Molecules. 2018; 23(4):740. https://doi.org/10.3390/molecules23040740
Chicago/Turabian StyleCai, Nan, Caixiu Liu, Zhihui Feng, Xinghai Li, Zhiqiu Qi, Mingshan Ji, Peiwen Qin, Wasim Ahmed, and Zining Cui. 2018. "Design, Synthesis, and SAR of Novel 2-Glycinamide Cyclohexyl Sulfonamide Derivatives against Botrytis cinerea" Molecules 23, no. 4: 740. https://doi.org/10.3390/molecules23040740