Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica
Abstract
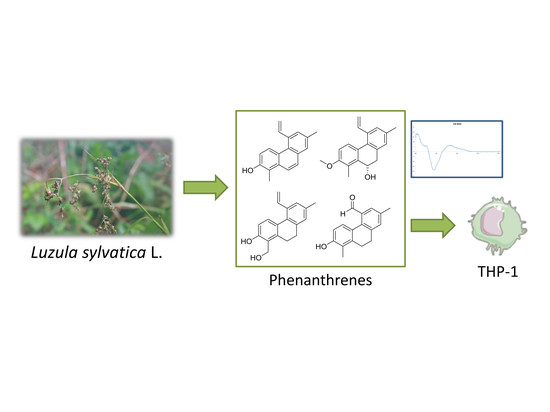
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Blood Leucocytes ROS Production
3.5. Cytotoxicity Evaluation of the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bús, C.; Tóth, B.; Stefkó, D.; Hohmann, J.; Vasas, A. Family Juncaceae: Promising source of biologically active natural phenanthrenes. Phytoch. Rev. 2018, 17, 833–851. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.-J.; Zhai, H.-F.; Zhang, B.; Duan, T.-X.; Huang, J.-M. Anxiolytic and sedative effects of dehydroeffusol from Juncus effusin mice. Planta Med. 2010, 77, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Menedez-Baceta, G.; Aceituno-Mata, L.; Molina, M.; Reyes-García, V.; Tardío, J.; Padro-de-Sanayana, M. Medicinal plants traditionally used in the northwest of the Basque Country (Biscay and Alava), Iberian Peninsula. J. Ethnoparmacol. 2014, 152, 113–134. [Google Scholar] [CrossRef]
- Li, H.-X.; Deng, T.-Z.; Chen, Y.; Feng, H.-J.; Yang, G.-Z. Isolation and identification of phenolic constituents from Juncus effusus. Acta Pharm. Sin. 2007, 42, 174–178. [Google Scholar]
- Della Greca, M.; Fiorentino, A.; Isidori, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Benzocoumarins from the rhizomes of Juncus acutus. Tetrahedron 2003, 59, 4821–4825. [Google Scholar] [CrossRef]
- Shan, C.-Y.; Ye, Y.-H.; Jiang, H.-F.; Zhang, J. Study on chemical constituents isolated from Juncus effusus. J. Chin. Med. Mat. 2008, 31, 374–376. [Google Scholar]
- Dong-Zhea, J.; Zhi-Daa, M.; Geroge, C.Y.C.; Munekazu, I.; Toshiyuki, T. Two p-coumaryl glycerides from Juncus effusus. Phytochemistry 1996, 41, 545–547. [Google Scholar] [CrossRef]
- Behery, F.A.A.; Naeem, Z.E.M.; Maatooq, G.T.; Amer, M.M.A.; Wen, Z.H.; Sheu, J.H.; Ahmed, A.F. Phenanthrenoides from Juncus acutus L., new natural lipopolysaccharide-inducible nitric oxide synthase inhibitors. Chem. Pharm. Bull. 2007, 55, 1264–1266. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhang, Y.; Ding, Y.-Y.; Liu, F.; Li, N. Cytotoxic and anti-inflammatory activities of phenanthrenes from the meduillae Juncus effusus L. Arch. Pharm. Res. 2016, 39, 154–160. [Google Scholar] [CrossRef]
- Kovács, A.; Vasas, A.; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A promising group of plant secondary metabolites. J. Nat. Prod. 2018, 81, 661–678. [Google Scholar]
- Dawe, A.; Pierre, S.; Tsala, D.E.; Habtemariam, S. Phytochemical constituents of Combretum Loefl. (combretaceae). Pharm. Crops 2013, 4, 38–59. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Phytochemical and antimicrobial investigations of stilbenoids and flavonoids isolated from three species of Combretaceae. Fitoterapia 2012, 83, 932–940. [Google Scholar] [CrossRef]
- Dumé, G.; Gauberville, C.; Mansion, D.; Rameau, J.-C. Flore Forestière Française 1; CNDF: Desmaures, QC, Canada, 2018; pp. 1774–1775. [Google Scholar]
- Tóth, B.; Chang, F.-R.; Tsong-Long, W.; Hwang, L.; Szappanos, Á.; Mándi, A.; Hunyati, A.; Tibor, K.; Jakab, G.; Hohmann, J.; et al. Screening of Luzula species native to the Carpathian Basin for anti-inflammatory activity and bioactivity-guided isolation of compounds from Luzula luzuloides (Lam.) Dandy & Wilmott. Fitoterapia 2017, 116, 131–138. [Google Scholar]
- Hu, J.; Lin, H.; Shen, J.; Lan, J.; Ma, C.; Zhao, Y.; Lei, F.; Xing, D.; Du, L. Developmental toxicity of orally administered pineapple leaf extract in rats. Food Chem. Toxicol. 2011, 49, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Vargaa, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef]
- Novakovic, M.; Djordjevic, I.; Todorovic, N.; Trifunovic, S.; Andjelkovic, B.; Mandic, B.; Jadranin, M.; Vuckovic, I.; Vajs, V.; Milosavljevic, S.; et al. New aurone epoxide and auronolignan from the heartwood of Cotinus coggygria Scop. Nat. Prod. Res. 2019, 33, 2837–2844. [Google Scholar] [CrossRef]
- Mizuno, M.; Yamashita, S.; Hashimoto, T. Enhancement of anti-inflammatory and anti-allergic activities with combination of luteolin and quercetin in in vitro co-culture system. Food Sci. Technol. Res. 2017, 23, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Seelinger, G.; Merfort, I.; Schempp, C. Anti-oxydant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Tóth, B.; Liktor-Busa, E.; Kúsz, N.; Szappanos, Á.; Mándi, A.; Kurtán, T.; Urbán, E.; Hohmann, J.; Chang, F.-R.; Vasas, A. Phenantherenes from Juncus inflexus with antimicrobial activity against methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2016, 79, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mogib, M. New 9,10-dihydrophenanthrenes derivatives from two Juncus species. Alex. J. Pharm. Sci. 2001, 15, 13–15. [Google Scholar]
- Dar, M.-Y.; Ara, T.; Akbar, S. A new prenylated coumarin from Daphne oleoides and its cytotoxic activity. Chem. Nat. Compd. 2019, 55, 5–7. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Tian, X. The components of Cacalia tangutica. B. Kor. Chem. Soc. 2004, 25, 1078–1080. [Google Scholar]
- Wang, X.-Y.; Ke, C.-Q.; Tang, C.-P.; Yuan, D.; Ye, Y. 9,10-Dihydrophenathrenes and Phenanthrenes from Juncus setchuensis. J. Nat. Prod. 2009, 72, 1209–1212. [Google Scholar] [CrossRef]
- Resnick, S.M.; Gibson, D.T. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: Structure and absolute stereochemistry of metabolites. Appl. Environ. Microbiol. 1996, 62, 3355–3359. [Google Scholar] [CrossRef] [Green Version]
- Della Greca, M.; Fiorentino, A.; Mangoni, L.; Molinaro, A.; Monaco, P.; Previtera, L. Cytotoxic 9,10-dihydrophenanthrenes from Juncus effusus L. Tetrahedron 1993, 49, 3425–3432. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Wang, Y.-L.; Zhai, H.-F.; Liao, Y.-J.; Zhang, B.; Huang, J.-M. Phenanthrenes from Juncus effusus with anxiolytic and sedative activities. Nat. Prod. Res. 2012, 26, 1234–1239. [Google Scholar] [CrossRef]
- Su, X.-H.; Yuan, Z.-P.; Li, C.-Y.; Zhong, Y.-J.; Du, H.-J.; Wen, Y.-Y.; Li, Y.-F.; Liang, B. Phenanthrenes from Juncus effusus. Planta Med. 2013, 79, 10447–10452. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Kosuge, Y.; Hamagami, H.; Ozaki, M.; Ishige, K.; Ito, Y.; Kitanaka, S. Chemical constituents isolated from Juncus effusus induce cytotoxicity in HT22 cells. J. Nat. Med. 2015, 69, 421–426. [Google Scholar] [CrossRef]
- Miles, D.H.; Bhattacharyya, J.; Mody, N.V.; Atwood, J.L.; Black, S.; Hedin, P.A. The structure of juncusol. A novel cytotoxic dihydrophenanthrene from the Estuarine marsh plant Juncus roemerianus. J. Am. Chem. Soc. 1977, 99, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.-P.; Guillot, J. Potential anti-inflammatory effects of Melaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother. Res. 2006, 5, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Ogéron, C.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D.; et al. Comparison of the anti-inflammatory and immunomodulatory mechanisms of two medicinal herbs: Meadowsweet (Filipendula ulmaria) and harpagophytum (Harpagophytum procumbens). Int. J. Plant Anim. Environ. Sci. 2019, 9, 145–163. [Google Scholar]
- Ramdani, L.-H.; Talhi, O.; Taibi, N.; Delort, L.; Decombat, C.; Silva, A.; Bachari, K.; Vasson, M.-P.; Caldefie-Chezet, F. Effects of Spiro-bisheterocycles on proliferation and apoptosis in human breast cancer cell lines. Anticancer Res. 2016, 36, 6399–6408. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |




| Compound | IC50 (µM) |
|---|---|
| 1 | >100 |
| 2 | 10 |
| 3 | 13 |
| 4 | 3 |
| 5 | 11 |
| 6 | 6 |
| 7 | 5 |
| 8 | 10 |
| 9 | >20 |
| Compound 4 | Compound 5 | Compound 8 | Compound 9 | |||||
|---|---|---|---|---|---|---|---|---|
| Position | δC type | δH (J in Hz) | δC type | δH (J in Hz) | δC type | δH (J in Hz) | δC type | δH (J in Hz) |
| 1 | 117.3, C | 126.8, C | 124.1, C | 121.7, C | ||||
| 2 | 151.1, C | 155.3, C | 157.1, C | 154.5, C | ||||
| 3 | 114.8, CH | 7.08, d (9.1) | 113.5, CH | 6.78, d (8.5) | 109.2, CH | 6.87, d (8.4) | 112.7, CH | 6.76, d (8.4) |
| 4 | 127.4 CH | 8.65, d (9.1) | 130.6, CH | 7.49, d (8.5) | 128.5, CH | 7.56, d (8.4) | 129.4, CH | 6.92, d (8.4) |
| 5 | 137.1 C | 135.1, C | 135.4 C | 140.1, C | ||||
| 6 | 130.6, CH | 7.45, s | 127.4, CH | 7.25, s | 128.3, CH | 7.26, s | 127.2, CH | 7.64, s |
| 7 | 134.9, C | 136.0, C | 136.7, C | 136.3, C | ||||
| 8 | 128.2, CH | 7.60, s | 127.6, CH | 7.00, s | 130.1, CH | 7.09, s | 133.1, CH | 7.30, s |
| 9 | 127.8, CH | 7.69, d (9.1) | 30.0, CH2 | 2.69, m | 38.2, CH2 | 3.15, dd (16, 2.8) | 29.1, CH2 | 2.78, m |
| 9’ | 2.95, dd (16, 3) | |||||||
| 10 | 122.8, CH | 7.89, d (9.1) | 25.1, CH2 | 2.69, m | 64.0, CH | 5.14, m | 25.5, CH2 | 2.82, m |
| 11 | 11.3, CH3 | 2.61, s | 60.2, CH2 | 5.01, s | 11.2, CH3 | 2.37, s | 11.8, CH3 | 2.30, s |
| 12 | 142.0, CH | 7.47, dd (17.3, 10.7) | 138.8, CH | 6.93, dd (17.4, 10.8) | 55.8, CH3 | 3.88, s | 193.6, CH | 10.07, s |
| 13 | 114.2, CH2 | 5.78, dd (17.3, 1.7) | 114.1, CH2 | 5.70, dd (17.4, 1.3) | 139.1, CH | 7.01, dd (17, 10) | 21.1, CH3 | 2.41, s |
| 13’ | 5.44, dd (10.7, 1.7) | 5.25 dd (10.8, 1.3) | ||||||
| 14 | 21.3, CH3 | 2.54, s | 21.2, CH3 | 2.37, s | 114.3, CH2 | 5.72, dd (17, 2) | ||
| 14’ | 5.28, dd (10, 2) | |||||||
| 15 | 21.1, CH3 | 2.37, s | ||||||
| 1a | 133.4, C | 138.4, C | 138.2, C | 139.5, C | ||||
| 4a | 125.8, C | 121.6, C | 125.4, C | 124.4, C | ||||
| 5a | 127.3, C | 131.1, C | 129.9, C | 136.7, C | ||||
| 8a | 132.0, C | 138.5, C | 132.9, C | 133.0, C | ||||
| OH | 4.94 brs | 5.12, brs | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gainche, M.; Ripoche, I.; Senejoux, F.; Cholet, J.; Ogeron, C.; Decombat, C.; Danton, O.; Delort, L.; Vareille-Delarbre, M.; Berry, A.; et al. Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica. Molecules 2020, 25, 2372. https://doi.org/10.3390/molecules25102372
Gainche M, Ripoche I, Senejoux F, Cholet J, Ogeron C, Decombat C, Danton O, Delort L, Vareille-Delarbre M, Berry A, et al. Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica. Molecules. 2020; 25(10):2372. https://doi.org/10.3390/molecules25102372
Chicago/Turabian StyleGainche, Maël, Isabelle Ripoche, François Senejoux, Juliette Cholet, Clémence Ogeron, Caroline Decombat, Ombeline Danton, Laetitia Delort, Marjolaine Vareille-Delarbre, Alexandre Berry, and et al. 2020. "Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica" Molecules 25, no. 10: 2372. https://doi.org/10.3390/molecules25102372