Thiophene Derivatives as Anticancer Agents and Their Delivery to Tumor Cells Using Albumin Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. MTT Screening Assay
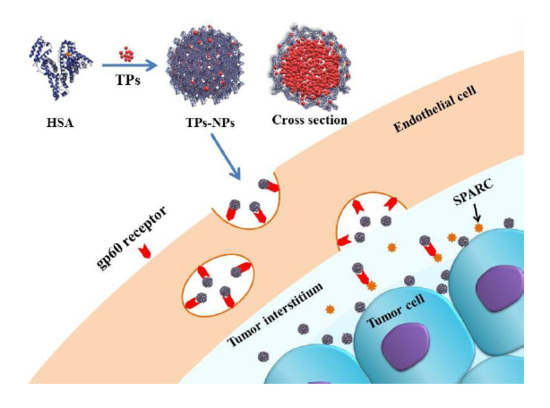
2.2. Synthesis of TPs-NPs
2.3. The Characterization of TP 5-NPs
2.4. Cellular Uptake In Vitro
2.5. Drug Release Kinetics of TP 5-NPs
2.6. Cytotoxicity of TP 5-NPs In Vitro
2.7. Mitochondrial Transmembrane Potential Assay
2.8. ROS Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture and MTT Screening Assay
4.2.2. Synthesis of TP 5-NPs
4.2.3. Synthesis of FITC-Labelled NPs
4.2.4. The Characterization of TP 5-NPs
4.2.5. Cellular Uptake of TP 5-NPs In Vitro
4.2.6. Drug Loading and Release of TP 5-NPs
4.2.7. Cytotoxicity of TP 5-NPs In Vitro
4.2.8. Mitochondrial Transmembrane Potential Assay
4.2.9. The Generation Level of Reactive Oxygen Species
4.2.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qu, N.; Sun, Y.T.; Xie, J.; Teng, L.S. Preparation and evaluation of in vitro self-assembling HSA nanoparticles for cabazitaxel. Anti-Cancer Agents Med. Chem. 2017, 17, 294–300. [Google Scholar] [CrossRef]
- Lu, H.X.; Noorani, L.; Jiang, Y.Y.; Du, A.W.; Stenzel, M.H. Penetration and drug delivery of albumin nanoparticles into pancreatic multicellular tumor spheroids. J. Mate Chem. 2017, 5, 9591–9599. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, W.W. Pd-Catalyzed intramolecular C-H addition to the cyano-group: Construction of functionalized 2,3-fused thiophene scaffolds. Org. Chem. Front. 2018, 5, 801–805. [Google Scholar] [CrossRef]
- Kesharwani, T.; Giraudy, K.A.; Morgan, J.L.; Kornman, C.; Olaitan, A.D. Green synthesis of halogenated thiophenes, selenophenes and benzo[b]selenophenes using sodium halides as a source of electrophilic halogens. Tetrahedron Lett. 2017, 58, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Manikandamathavan, V.M.; Thangaraj, M.; Weyhermuller, T. Novel mononuclear Cu (II) terpyridine complexes: Impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line. Eur. J. Med. Chem. 2017, 135, 434–446. [Google Scholar] [CrossRef]
- Aleksić, M.; Bertoša, B.; Nhili, R. Anilides and quinolones with nitrogen-bearing substituents from benzothiophene and thienothiophene series: Synthesis, photochemical synthesis, cytostatic evaluation, 3D-derived QSAR analysis and DNA-binding properties. Eur. J. Med. Chem. 2014, 71, 267–281. [Google Scholar] [CrossRef]
- Véras Of Aguiar, A.C.; Of Moura, R.O.; Bezerra Mendonça, J.F.; de Oliveira Rocha, H.A.; Gomes Câmara, R.B.; Dos Santos Carvalho Schiavon, M. Evaluation of the antiproliferative activity of 2-amino thiophene derivatives against human cancer cells lines. Biomed. Pharmacother. 2016, 84, 403–414. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-nano approach: Enabling the translation of metal nanomaterials to clinics. Bioconjug. Chem. 2017, 29, 4–16. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Brit. J. Cancer 2008, 99, 392. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Z.G.; Zhou, C.G.; Zhu, J.; Lee, R.J.; Teng, L.S. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef]
- Warlick, H.; Michaelis, K.; Balthasar, S.; Strebhardt, K.; Kreuter, J.; Langer, K. Highly specific HER2-mediated cellular uptake of antibody-modified nanoparticles in tumor cells. J. Drug Target 2004, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 22–30. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Li, F.; Zheng, C.L.; Xin, J.; Chen, F.B.; Ling, H.; Sun, L.L.; Webster, T.J.; Ming, X.; Liu, J.P. Enhanced tumor delivery and antitumor response of doxorubicin-loaded albumin nanoparticles formulated based on a Schiff base. Int. J. Nanomed. 2016, 11, 3875–3890. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery-new applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Yang, X.W.; Yang, S.; Chai, H.Y.; Yang, Z.G.; Lee, R.J.; Liao, W.W.; Teng, L.S. A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferrin-conjugated liposomes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Moriyama, Y.; Takeda, K. Secondary structural changes of intact and disulfide bridges-cleaved human serum albumins in thermal denaturation up to 130 °C—Additive effects of sodium dodecyl sulfate on the changes. J. Oleo Sci. 2017, 66, 521–529. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Ramamurthi, A. Nanotherapeutics to modulate the compromised micro-environment for lung cancers and chronic obstructive pulmonary disease. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Kommineni, N.; Khan, W. Exploring the potential of nanotherapeutics in targeting tumor microenvironment for cancer therapy. Pharmacol. Res. 2017, 126, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressler, C.; Beuthan, J.; Mueller, G.; Zabarylo, U.; Minet, O. Fluorescence imaging of heat-stress induced mitochondrial long-term depolarization in breast cancer cells. J. Fluoresc. 2006, 16, 689–695. [Google Scholar] [CrossRef]
- Dressler, C.; Minet, O.; Zabarylo, U.; Beuthan, J. Fluorescence imaging of mitochondrial long-term depolarization in cancer cells exposed to heat stress. IGI Glob. 2009, 2. [Google Scholar] [CrossRef]
- Wang, M.Q.; Lee, R.J.; Bi, Y.; Li, L.L.; Yan, G.D.; Lu, J.H.; Meng, Q.F.; Teng, L.S.; Xie, J. Transferrin-conjugated liposomes-loaded with novel dihydroquinoline derivatives as potential anticancer agents. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Roberto, M.; Michael, E.; Jessica, R.H.; Claudia, C.; Francesca, T.; Jonathan, O.M.; Kelly, A.H.; Donato, C.; Giosue’, C.; Isabella, R.; et al. Design and development of biomimetic nanovesicles using a microfluidic approach. Adv. Mater. 2018, 30, e1702749. [Google Scholar]
- Chen, L.; Li, L.F.; Zhang, H.; Liu, W.F.; Yang, Y.Z.; Liu, X.G.; Xu, B.S. Magnetic thermosensitive core/shell microspheres: Synthesis, characterization and performance in hyperthermia and drug delivery. RSC Adv. 2014, 4, 46806–46812. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Li, L.F.; Yang, Y.Z.; Liu, X.G.; Xu, B.S. Thermoresponsive hollow magnetic microspheres with hyperthermia and controlled release properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gilmore, D.; Colson, Y.L. Tumor targeted nanoparticles: A modern day trojan horse. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 10–11. [Google Scholar] [CrossRef]
- Qu, N.; Lee, R.J.; Sun, Y.T.; Cai, G.S.; Wang, J.Y.; Wang, M.Q.; Lu, J.H.; Meng, Q.F.; Teng, L.R.; Wang, D.; et al. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int. J. Nanomed. 2016, 11, 3451–3459. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.N.; Wei, Z.W.; Chen, H.G.; Liu, J.L.; Guo, J.J.; Cao, M.; Wen, T.Q.; Shi, L.Y. Folic acid-functionalized up-conversion nanoparticles: Toxicity studies in vivo and in vitro and targeted imaging applications. Nanoscale 2014, 6, 8878–8883. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.X.; Gai, Z.; Hong, K.L.; Banerjee, P.; Zhou, S.Q. Magnetic/NIR-responsive drug carrier, multicolor cell imaging, and enhanced photothermal therapy of gold-capped magnetite-fluorescent carbon hybrid nanoparticles. Nanoscale 2015, 7, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Park, C.; Jeong, J.W.; Kim, M.J.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, G.Y.; Choi, B.T.; Choi, Y.H.; et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen species-mediated mitochondrial death pathway. Int. J. Oncol. 2013, 42, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.K.; She, T.T.; Wang, L.X.; Su, Y.H.; Qu, L.; Gao, Y.K.; Xu, S.; Cai, S.Q.; Shou, C.C. Daucosterol inhibits cancer cell proliferation by inducing autophagy through reactive oxygen species-dependent manner. Life Sci. 2015, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds TP 5 and TP 5-NPs are available from the authors. |









| Solvent | Solubility |
|---|---|
| Deionized water | 26.36 μg/mL |
| PBS buffered solution (pH 7.4) | 27.64 μg/mL |
| Ethanol | 2.48 mg/mL |
| Ethyl acetate | >6.80 mg/mL |
| Dichloromethane | >13.00 mg/mL |
| Temperature (°C) | Calculated Concentration (μg/mL) | Detected Concentration (μg/mL) | RSD (%) |
|---|---|---|---|
| 25.0 ± 0.50 | 200.0 ± 0.50 | 201.18 | 0.27 |
| 200.53 | |||
| 199.88 | |||
| 40.0 ± 0.50 | 200.0 ± 0.50 | 202.66 | 0.55 |
| 200.05 | |||
| 202.01 | |||
| 60.0 ± 0.50 | 200.0 ± 0.50 | 201.19 | 0.84 |
| 202.28 | |||
| 202.93 |
| Number | TP 5 Concentration (mg/mL) | Temperature (°C) | Size (nm) | PDI | ξ Potential (mV) |
|---|---|---|---|---|---|
| 1 | 2.0 ± 0.10 | 25.0 ± 0.50 | 425.9 ± 0.34 | 0.748 ± 0.05 | −22.8 ± 0.05 |
| 2 | 2.0 ± 0.10 | 40.0 ± 0.50 | 217.9 ± 0.83 | 0.051 ± 0.06 | −23.6 ± 0.09 |
| 3 | 3.0 ± 0.10 | 40.0 ± 0.50 | 177.4 ± 1.80 | 0.156 ± 0.16 | −22.2 ± 1.05 |
| 4 | 4.0 ± 0.10 | 40.0 ± 0.50 | 166.8 ± 0.09 | 0.143 ± 0.14 | −25.1 ± 1.21 |
| 5 | 5.0 ± 0.10 | 40.0 ± 0.50 | 1084.0 ± 0.10 | 0.817 ± 0.09 | −26.5 ± 1.30 |
| 6 | 4.0 ± 0.10 | 60.0 ± 0.50 | 247.5 ± 2.03 | 0.169 ± 0.05 | −22.2 ± 2.09 |
| 7 | 5.0 ± 0.10 | 60.0 ± 0.50 | 202.6 ± 2.64 | 0.187 ± 0.03 | −22.6 ± 1.26 |
| 8 | 6.0 ± 0.10 | 60.0 ± 0.50 | 205.4 ± 3.44 | 0.154 ± 0.17 | −23.2 ± 3.07 |
| 9 | 7.0 ± 0.10 | 60.0 ± 0.50 | 47381.1 ± 3.18 | 1.000 ± 0.66 | −24.7 ± 1.03 |
| Number | TPs Concentration (mg/mL) | Temperature (°C) |
|---|---|---|
| 1 | 2.0 ± 0.10 | 25.0 ± 0.50 |
| 2 | 2.0 ± 0.10 | 40.0 ± 0.50 |
| 3 | 3.0 ± 0.10 | 40.0 ± 0.50 |
| 4 | 4.0 ± 0.10 | 40.0 ± 0.50 |
| 5 | 5.0 ± 0.10 | 40.0 ± 0.50 |
| 6 | 4.0 ± 0.10 | 60.0 ± 0.50 |
| 7 | 5.0 ± 0.10 | 60.0 ± 0.50 |
| 8 | 6.0 ± 0.10 | 60.0 ± 0.50 |
| 9 | 7.0 ± 0.10 | 60.0 ± 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, G.; Wang, S.; Zhao, L.; Sun, Y.; Yang, D.; Lee, R.J.; Zhao, M.; Zhang, H.; Zhou, Y. Thiophene Derivatives as Anticancer Agents and Their Delivery to Tumor Cells Using Albumin Nanoparticles. Molecules 2019, 24, 192. https://doi.org/10.3390/molecules24010192
Cai G, Wang S, Zhao L, Sun Y, Yang D, Lee RJ, Zhao M, Zhang H, Zhou Y. Thiophene Derivatives as Anticancer Agents and Their Delivery to Tumor Cells Using Albumin Nanoparticles. Molecules. 2019; 24(1):192. https://doi.org/10.3390/molecules24010192
Chicago/Turabian StyleCai, Guangsheng, Simiao Wang, Lang Zhao, Yating Sun, Dongsheng Yang, Robert J. Lee, Menghui Zhao, Huan Zhang, and Yulin Zhou. 2019. "Thiophene Derivatives as Anticancer Agents and Their Delivery to Tumor Cells Using Albumin Nanoparticles" Molecules 24, no. 1: 192. https://doi.org/10.3390/molecules24010192