Abstract
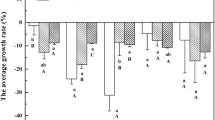
Arsenic speciation in natural surface-water systems can be highly impacted through biological processes that result in non-thermodynamically predicted species to dominate the system. In laboratory experiments, arsenate reduction by a freshwater cyanobacterium exhibited saturation kinetics increasingly inhibited by elevating solution phosphate concentrations. Approximately 100% arsenate reduction occurred by days 4, 7, and 10 in the low (0.35 µm), middle (3.5 µm), and high (35 µm) phosphate treatments, respectively, with maximum arsenate reduction rates ranged from 0.013 μmol As g C−1 day−1 in the high-phosphate treatment to 0.398 μmol As g C−1 day−1 in the low-phosphate treatment. Saturation kinetic models were utilized to evaluate the impact of cell growth and arsenate-phosphate uptake competition on arsenate reduction rates by the cyanobacterium. Results showed reduced arsenicals dominate arsenic speciation once growth reached steady state, indicating reduced arsenicals may dominate natural systems, even when considering conservatively high, abiotic arsenic reoxidation.




Similar content being viewed by others
References
Agency for Toxic Substances and Disease Registry (ATSDR). (2000). Toxicological profile for arsenic. Atlanta: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Allen, M. M. (1968). Simple conditions for growth of unicellular blue-green algae on plates. Journal of Phycology, 4, 1–3.
Andreae, M. O. (1979). Arsenic speciation in seawater and interstitial waters: The influence of biological-chemical interactions on the chemistry of a trace element. Limnology and Oceanography, 24(3), 440–452.
Benson, A. A. (1989). Arsonium compounds in algae. Proceedings of the National Academy of Sciences of the United States of America, 86, 6131–6132.
Blum, J. S., et al. (1998). Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: Two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Archives of Microbiology, 171, 19–30.
Droop, M. R. (1974). The nutrient status of algal cells in continuous culture. Journal of the Marine Biological Association of the United Kingdom, 54, 825–855.
Eaton, A. D., Clesceri, L. S., & Greenberg, A. E. (eds). (1995). Standard methods for the examination of water and wastewater, 19th ed. 3114 C. Continuous hydride generation/atomic absorption spectrometric method. Washington, DC: American Public Health Association.
Gallagher, P. A., et al. (2001). Speciation and preservation of inorganic arsenic in drinking water sources using EDTA with IC separation and ICP0MS detection. Journal of Environmental Monitoring, 3, 371–376.
Golden, J. W., & Yoon, H.-S. (2003). Heterocycst development in Anabaena. Current Opinion in Microbiology, 6, 5557–5563.
Hasegawa, H., et al. (2001). Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere, 43, 265–272.
Hellweger, F. L., et al. (2003). Greedy algae reduce arsenate. Limnology and Oceanography, 48(6), 2275–2288.
Jain, C. K., & Ali, I. (2000). Arsenic: Occurrence, toxicity and speciation techniques. Water Resources, 34, 4304–4312.
Kaise, T., et al. (1999). Accumulation of arsenic in a unicellular alga Chlamydomonas reinhardtii. Applied Organometallic Chemistry, 13, 107–111.
Knauer, K., & Hemond, H. (2000). Accumulation and reduction of arsenate by the freshwater green alga Chlorella sp. (chlorophyta). Journal of Phycology, 36, 506–509.
Kuroiwa, T., et al. (1994). Biomethylation and biotransformation of arsenic in a freshwater food chain: Green alga (Chlorella vulgaris) > shrimp (Neocaridina denticulata) > killifish (Oryzias latipes). Applied Organometallic Chemistry, 8, 325–333.
Lai, V. W.-M., et al. (1997). The characterization of arsenosugars in commercially available algal products including a Nostoc species of terrestrial origin. Applied Organometallic Chemistry, 11, 797–803.
Levine, S. N., & Schindler, D. W. (1999). Influence of nitrogen to phosphorus supply ratios and physicochemical conditions on cyanobacteria and phytoplankton species composition in the Experimental Lakes Area, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 56, 451–466.
Markley, C. T. (2004). Arsenate uptake, sequestration and reduction by a freshwater cyanobacterium: A potential biologic control of arsenic in South Texas (pp. 104). College Station: Department of Geology and Geophysics, Texas A&M University.
Markley, C. T., & Herbert, B. E. (2009). Arsenic risk assessment: The importance of speciation in different hydrologic systems. Water, Air, and Soil Pollution, doi:10.1007/s11270-009-0052-6.
Mirimanoff, N., & Wilkinson, K. J. (2000). Regulation of Zn accumulation by a freshwater gram-positive bacterium (Rhodococcus opacus). Environmental Science & Technology, 34, 616–622.
Oremland, R. S., & Stoltz, J. F. (2003). The ecology of arsenic. Science, 300, 939–944.
Paerl, H. W. (1988). Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnology and Oceanography, 33(4, part 2), 823–847.
Rhee, G.-Y. (1973). A continuous culture study of phosphate uptake, growth rate and polyphosphate in Scenedesmus sp. Journal of Phycology, 9, 495–506.
Smedley, P. L., & Kinniburgh, D. G. (2002). The review of source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Sohrin, Y., et al. (1997). Arsenic biogeochemistry affected by eutrophication in Lake Biwa, Japan. Environmental Science & Technology, 31, 2712–2720.
Sterner, R. W., & Elser, J. J. (2002). Ecological stoichiometry. Princeton: Princeton University Press.
Takahashi, A., et al. (2001). Some characteristics of arsenate transport in a marine cyanobacterium, Synechococcus sp. Applied Organometallic Chemistry, 15, 291–298.
Theil, T. (1988). Phosphate transport and arsenate resistance in the cyanobacterium Anabaena variablis. Journal of Bacteriology, 170, 1143–1147.
Tukai, R., et al. (2002). Measurement of arsenic species in marine macroalgae by microwave-assisted extraction and high performance liquid chromatography-inductively coupled plasma mass spectrometry. Analytica Chimica Acta, 457, 173–185.
Vahter, M., & Concha, G. (2001). Role of metabolism in arsenic toxicity. Pharmacology and Toxicology, 89, 1–5.
Acknowledgements
I would like to acknowledge the various sources of funding I have received. Thanks to the Texas Water Resource Institute for the 2003–2004 W. C. Mills Fellowship. Thanks to the College of Engineering for the 2003–2004 Graduate Assistantship in Areas of National Need (GAANN) Fellowship funded by the US Department of Education. Final thanks to the Texas Advanced Research Program: Project No. 010366-0364-1999. Research for this study was conducted while all authors were enrolled and/or employed at Texas A&M University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markley, C.T., Herbert, B.E. Modeling Phosphate Influence on Arsenate Reduction Kinetics by a Freshwater Cyanobacterium. Environ Model Assess 15, 361–368 (2010). https://doi.org/10.1007/s10666-009-9212-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10666-009-9212-8