Abstract
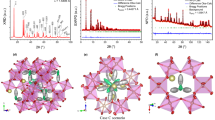
The crystal structure of aP21/a polymorph of sapphirine (a=11.286(3),b=14.438(2),c=9.957(2) Å, β=125.4(2) °) of composition [Mg3.7Fe 2+0.1 Al4.1- Fe 3+0.1 ]IV[Si1.8Al4.2]IVO20 was refined using structure factors determined by both neutron and x-ray diffraction methods to conventionalR factors of 0.067 and 0.031. respectively, forF obs>2σ. The results of the two refinements agree reasonably well, but a half-normal probability plot (Abrahams, 1974) comparing the two data sets indicates that the pooled standard deviations of the atomic coordinates have been underestimated by a factor of two.
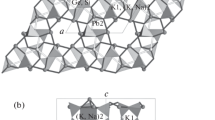
The structure of sapphirine, solved initially by Moore (1969), consists of cubic closest packed oxygens with octahedral and predominantly tetrahedral layers alternately stacked along [100]. The layer in which 70% of the octahedral sites are occupied has an Mg-Al distribution characterized by Mg-rich octahedra sharing edges mainly with Al-rich octahedra. Mean octahedral bond lengths correlate well with Al occupancy determined by neutron site refinement if the relative number of shared octahedral edges is taken into account (see Table 1).
The predominantly tetrahedral layer has 10% of the octahedral sites occupied by Al and 30% of the tetrahedral sites occupied by Al-Si in the ratio 2.33∶1. There are single chains of Al-Si tetrahedra parallel toz with corner-sharing wing tetrahedra (T5 andT6) on either side in the (100) plane. The meanT-O distance is highly correlated with Al occupancy, XAl, as determined from the neutron site refinement:
Details of the neutron refinement are summarized below.
Similar content being viewed by others
References
Abrahams, S.C.: Systematic error differences between two refined sets of position coordinates for Na3PO3CO2 · 6H2O. Acta Cryst.B28, 2886–2887 (1972)
Abrahams, S.C.: The reliability of crystallographic structural information. Acta Cryst.B30, 261–268 (1974)
Abrahams, S.C., Keve, E.T.: Normal probability plot analysis of error in measured and derived quantities and standard deviations. Acta Cryst.A27, 157–165 (1971)
Bøggild, O.B.: The mineralogy of Greenland. Meddel. Grønland149, 192–194 (1953)
Brown, G.E., Hamilton, W.D., Prewitt, C.T., Sueno, S.: Neutron diffraction study of Al/Si ordering in sanidine: a comparison with X-ray diffraction data. In: The Feldspars (W.S. MacKenzie and J. Zussman, eds.), pp. 68–80. Manchester University Press 1974
Brown, I.D., Wu, K.K.: Empirical parameters for calculating cationoxygen bond valences. Acta Cryst.B32, 1957–1959 (1976)
Burnham, C.W.: Refinement of the crystal structure of sillimanite. Z. Krist.118, 127–148 (1963)
Charlu, T.V., Newton, R.C., Kleppa, O.J.: Enthalpies of formation at 970 ° K of compounds in the system MgO-Al2O33-SiO2 from high temperature solution calorimetry. Geochim. Cosmochim. Acta39, 1487–1497 (1975)
Cohen, J.P., Ross, F.K., Gibbs, G.V.: An X-ray and neutron diffraction study of hydrous low cordierite. Am. Mineral.62, 67–78 (1977)
Coppens, P.: Some implications of combined X-ray and neutron diffraction studies. Acta Cryst.B30, 255–261 (1974)
Finger, L.W.: Determination of cation distribution by least-squares refinement of single crystal X-ray data. Ann. Rep. Dir. Geophys. Lab. Year Book67, 211–217 (1969)
Finger, L.W., Haxen, R.M., Yagi, T.: High-pressure crystal structures of the spinel polymorphs of Fe2SiO4 and Ni2SiO4. Ann. Rep. Dir. Geophys. Lab. Year Book76, 504–505 (1977)
Hamilton, W.C., Abrahams, S.C.: Normal probability plot analysis of small samples. Acta Cryst.A28, 215–218 (1972)
Harlow, G.E., Brown, G.E., Hamilton, W.C.: Neutron diffraction study of Amelia low albite (abstr.). Trans. Am. Geophys. Union54, 497 (1973)
Hawthorne, F.C., Grundy, H.D.: The crystal chemistry of the amphiboles: IV. X-ray neutron refinements of the crystal structure of tremolite. Can. Mineral.14, 334–345 (1976)
Hazen, R.M., Finger, L.W.: Crystal structure and compositional variation of Angra Dos Reis fassaite. Earth Planet. Sci. Lett.35, 357–362 (1977)
Herd, R.K.: The Petrology of the Sapphirine-Bearing and Associated Rocks of the Fiskenaesset Complex, West Greenland. Ph.D. Thesis, University of London 1972
Higgins, J.B.: Crystal Chemistry, Cation Ordering and Polymorphism in Sapphirine. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA 1978
Higgins, J.B., Ribbe, P.H., Herd, R.K.: Sapphirine I. Crystal chemical contributions. Contrib. Mineral. Petrol.68, 349–356 (1979)
Higgins, J.B., Ribbe, P.H., Ross, F.K.: A neutron diffraction study of sapphirine (abstr.). Trans. Am. Geophys. Union58, 522 (1977)
International Tables for X-ray Crystallography, Vol. 4. Birmingham: Kynoch Press 1974
Moore, P.B.: The crystal structure of sapphirine. Am. Mineral.54, 31–49 (1969)
Morimoto, N., Tokonami, M., Watanabe, M., Koto, K.: Crystal structures of three polymorphs of Co2SiO4. Am. Mineral.59, 475–485 (1974)
Peacor, D.R.: The crystal structure of CoGeO3. Z. Krist.126, 299–306 (1968)
Petersen, J.L., Dahl, L.F., Williams, J.M.: Neutron diffraction studies of the metal-hydrogen-metal bond. I. The symmetric, bent, three center, two-electron molybdenum-hydrogen-molybdenum bond in β-hydrido-β-dimethylphosphido-bis (N5-cyclopentadienyldi-carbonyl-molybdenum), Mo2(N5C5H5)2(CO)4-(β2-H)(μ2-P(CH3)2). J. Am. Chem. Soc.96, 6610–6620 (1974)
Prince, E., Donnay, G., Martin, R.F.: Neutron diffraction refinement of an ordered orthoclase structure. Am. Mineral.58, 500–507 (1973)
Ribbe, P.H., Gibbs, G.V.: Crystal structures of the humite minerals: III. Mg/Fe ordering in humite and its relation to other ferromagnesian silicates. Am. Mineral.56, 1155–1173 (1971)
Robinson, K., Gibbs, G.V., Ribbe, P.H.: Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science172, 567–570 (1971)
Roziere, J., Williams, J.M.: The hydrated proton H+(H2O)n. IV. A high-precision neutron diffraction study of the diaquohydrogen ion, (H2O·H·0H2)+ intrans-dichlorobis(ethylenediamine)-cobalt(III) chloride hydrochloride dihydrate. Inorg. Chem.15, 1174–1178 (1976)
Sahama, Th.G., Lehtinen, M., Rehtijarvi, P., v. Knorring, O.: Properties of sapphirine. Ann. Acad. Sci. Fenn.,Series A. III, 1–24 (1974)
Seifert, F.: Stability of sapphirine: A study of the aluminous part of the system MgO-Al2O3-SiO2-H2O. J. Geol.82, 173–204 (1974)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst.A32, 751–767 (1976)
Shannon, R.D., Prewitt, C.T.: Effective ionic radii in oxides and fluorides. Acta Cryst.B25, 925–946 (1969)
Smith, J.V.: Feldspar Minerals. I. Crystal Structure and Physical Properties. Berlin, Heidelberg, New York: Springer 1974.
Tables of Normal Probability Functions, National Bureau of Standards, Applied Mathematics Series No. 23. Washington: U.S. Government Printing Office 1953
Tossell, J.A., Gibbs, G.V.: The use of molecular-orbital calculations on model systems for the prediction of bridging-bondangle variations in siloxanes, silicates, silicon nitrides and silicon Sulfides. Acta Cryst.A34, 463–472 (1978)
Wenk, H.R., Raymond, K.N.: Four new structure refinements− of olivine. Z. Krist.137, 86–105 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higgins, J.B., Ribbe, P.H. Sapphirine II. Contr. Mineral. and Petrol. 68, 357–368 (1979). https://doi.org/10.1007/BF01164520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01164520