Abstract
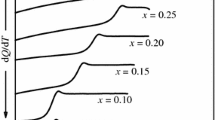
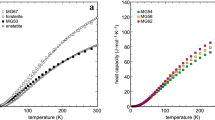
Enthalpies and heat capacities of glasses and of stable liquids in the system NaAlSi3O8-CaAl2Si2O8 have been measured by drop and differential scanning calorimetry. Within experimental error, values of C p and of H T 300 of three intermediate compositions fall on straight line interpolations between the end members for both liquids and glasses, indicating that excesses in true and in mean heat capacities [(H T −H 300)/(T−300)] are small or absent. A value for the heat capacity of the An100 liquid component can therefore be derived, and is probably a better estimate than that based on measurements on the pure substance alone. On the assumption of zero excess heat capacity in this system, heats of mixing in the stable liquids are equal to those measured in the glasses by solution calorimetry, and can be as negative as -2 kcal mol−1.
Heat capacities of solids and glasses in the Ab-An system are similar and do not vary greatly with composition. The C P's of the liquids, however, increase strongly with An content, suggesting major structural changes take place across the binary.
Similar content being viewed by others
References
Arndt J, Häberle F (1973) Thermal expansion and glass transition temperatures of synthetic glasses of plagioclase-like compositions. Contrib Mineral Petrol 39:175–183
Bacon CR (1977) High-temperature heat content and heat capacity of silicate glasses: experimental demonstration and a model for calculation. Am J Sci 277:109–135
Bottinga Y, Weill DF, Richet P (1981) Thermodynamic modeling of silicate melts. In: RC Newton, A Navrotsky, BJ Wood (eds) Thermodynamics of Minerals and Melts, Springer-Verlag, New York, pp 207–245
Bowen NL (1913) The melting phenomena of the plagioclase feldspars. Am J Sci, ser 4, 35:577–599
Bowen NL (1915) The crystallization of haplobasaltic, haplodioritic, and related magmas. Am J Sci, ser 4, 40:161–185
Carmichael ISA, Nicholls J, Spera FJ, Wood BJ, Nelson SA (1977) High-temperature properties of silicate liquids: applications to the equilibration and ascent of basic magma. Philos Trans R Soc London (A) 286:373–421
DeJong BHWS, Brown GE (1980) The polymerization of silicate and aluminate tetrahedra in glasses, melts, and aqueous solutions — I. Electronic structure of H6Si2O7, H6AlSiO 1/s-7 , and H6Al2O 2−7 . Geochim Cosmochim Acta 44:491–511
Ditmars DA, Douglas TB (1971) Measurement of the relative enthalpy of pure α-Al2O3 (NBS heat capacity and enthalpy standard reference material no. 720) from 273 to 1173 K. J Res NBS 75A:401–420
Ferrier A (1968) Mesure de l'enthalpie du diopside synthéthique entre 298 et 1885 K. C R Acad Sci Paris 267C:101–103
Ferrier A (1969) Etude expérimentale de l'enthalpie du l'anorthite synthétique entre 298 et 1950 K. C R Acad Sci Paris 268C:951–954
Haas JL, Fisher JR (1976) Simultaneous evaluation and correlation of thermodynamic data. Am J Sci 276:525–545
Haggerty JS, Cooper AR, Heasley JH (1968) Heat capacity of three inorganic glasses and supercooled liquids. Phys Chem Glasses 9:47–51
Henry DJ, Navrotsky A, Zimmermann HD (1982) Thermodynamics of plagioclase-melt equilibria in the system albite-anorthitediopside. Geochim Cosmochim Acta 46:381–391
Kelley KK (1960) High-temperature heat content, heat capacity, and entropy data for the elements and inorganic compounds. US Bur Mines Bull 584
Krupka KM, Robie RA, Hemingway BS (1979) High-temperature heat capacities of corundum, periclase, anorthite, CaAl2Si2O8 glass, muscovite, pyrophyllite, KAlSi3O8 glass, grosular, and NaAlSi3O8 glass. Am Mineral 64:86–101
Kushiro I, Schairer JF (1970) Diopside solid solutions an the system diopside-anorthite-albite at 1 atm and at high pressures. Carnegie Inst Washington Yearb 68:222–226
Kushiro I (1973) The system diopside-anorthite-albite: determination of composition of coexisting phases. Carnegie Inst Washington Yearb 72:502–507
Maier CG, Kelley KK (1932) An equation for the representation of high temperature heat content data. J Am Ceram Soc 54:3243–3345
Moore J, Sharp DE (1958) Note on the calculation of effect of temperature and composition on specific heat of glass. J Am Ceram Soc 41:461–463
Moynihan CT, Easted AJ, Tran DC, Wilder JA, Donovan EP (1976) Heat capacity and structural relaxation of mixed-alkali glasses
Murphy WM (1977) An experimental study of solid-liquid equilibria in the albite-anorthite-diopside system. MS thesis, University of Oregon, Eugene
Navrotsky A, Fukuyama H, Davies PK (1982) Calorimetric study of crystalline and glassy high pressure phases. High Pressure Res in Geophysics (in press)
Navrotsky A, Hon R, Weill DF, Henry DJ (1980) Thermochemistry of glasses and liquids in the systems CaMgSi2O6- CaAl2Si2O8-NaAlSi3O8, SiO2-CaAl2Si2O8-NaAlSi3O8- and SiO2-Al2O3-CaO-Na2O. Geochim Cosmochim Acta 44:1409–1423
Osborn EF (1942) The system CaSiO3-diopside-anorthite. Am J Sci 240:751–788
Riebling EF (1960) Structure of aluminosilicate melts containing at least 50 mole% SiO2at 1500° C. J Chem Phys 44:2857–2865
Richet P, Bottinga Y (1980) Heat capacity of liquid silicates: new measurements on NaAlSi3O8 and K2Si4O9. Geochim Cosmochim Acta 44:1535–1541
Robie RA, Hemingway BS, Fisher JR (1978) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 pascals) pressure and at higher temperatures. US Geol Surv Bull 1452
Robie RA, Hemingway BS, Wilson WH (1978) Low-temperature heat capacities and entropies of feldspar glasses and of anorthite. Am Mineral 63:109–123
Schairer JF, Yoder HS (1960) The nature of residual liquids from crystallization, with data on the system nepheline-diopside-silica. Am J Sci 258A:273–283
Schweite HE, Ziegler G (1955) Beitrag zur spezifischen Wärme der Gläser. Glastech Ber 28:137–146
Sharp DE, Ginther LB (1951) Effect of composition and temperature on the specific heat of glass. J Am Ceram Soc 34:260–271
Stebbins JF, Carmichael ISE, Weill DF (1981) Heats of fusion of diopside and sanidine and high pressure volume changes in aluminous silicate liquids. Geol Soc Am, Abstr Progr 13:560
Taylor M, Brown GE (1979) Structure of mineral glasses — I. The feldspar glasses NaAlSi3O8, KAlSi3O8, CaAl2Si2O8. Geochim Cosmochim Acta 43:61–75
Vergano PJ, Hill DC, Uhlmann DR (1967) Thermal expansion of feldspar glasses. J Amer Ceram Soc 50:59–60
Weill DF, Hon R, Navrotsky A (1980) The igneous system CaMgSi2O6-CaAl2Si2O8-NaAlSi3O8: variations on a classic theme by Bowen. In: Physics of magmatic processes. Princeton University Press, Princeton, New York, pp 49–92
Weill DF, Stebbins JF, Hon R, Carmichael ISE (1980) The enthalpy of fusion of anorthite. Contrib Mineral Petrol 74:95–102
White WP (1919) Silicate specific heats. Am J Sci 47:1–44
Winkelmann A (1893) Über die spezifische Wärme verschiedener zusammengesetzter Gläser. Ann Phys Chem 49:401–420
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stebbins, J.F., Weill, D.F., Carmichael, I.S.E. et al. High temperature heat contents and heat capacities of liquids and glasses in the system NaAlSi3O8-CaAl2Si2O8 . Contr. Mineral. and Petrol. 80, 276–284 (1982). https://doi.org/10.1007/BF00371357
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371357