High-Pressure Synthesis and Chemical Bonding of Barium Trisilicide BaSi3
Abstract
:1. Introduction
2. Materials and Methods
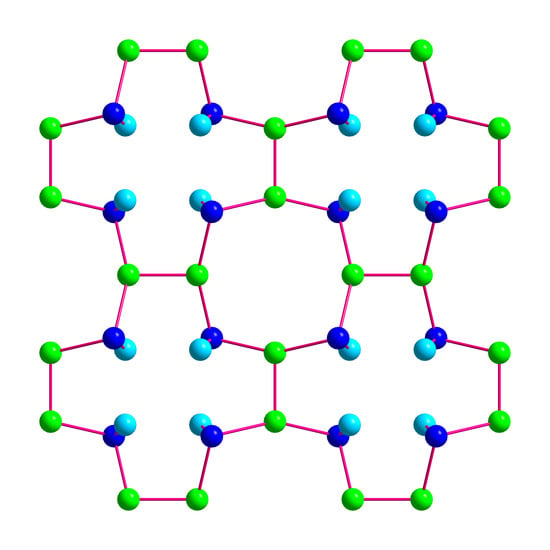
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zintl, E.; Brauer, G.Z. Über die Valenzelektronenregel und die Atomradien unedler Metalle in Legierungen. Phys. Chem. B 1933, 20, 245–271. [Google Scholar] [CrossRef]
- Zintl, E. Intermetallische Verbindungen. Angew. Chem. 1939, 52, 1–6. [Google Scholar] [CrossRef]
- Widera, A.; Schäfer, H. Darstellung und Kristallstruktur des Ba2Si. Z. Naturforsch. 1976, 31, 1434–1435. [Google Scholar] [CrossRef]
- Eisenmann, B.; Janzon, K.H.; Schäfer, H.; Weiss, A. Zur Kenntnis von Ba3Si4. Z. Naturforsch. 1969, 24, 457–458. [Google Scholar] [CrossRef]
- Gladyshevskii, E.I. The crystal structure of BaSi2 and CeGe2. Dopovidi Akademii Nauk Ukrains’koi RSR 1959, 1959, 294–297. [Google Scholar]
- San-Miguel, A.; Toulemonde, P. High-pressure properties of group IV clathrates. Adv. High Pressure Res. 2005, 25, 159–185. [Google Scholar] [CrossRef]
- Flores-Livas, J.A.; Debord, R.; Botti, S.; San-Miguel, A.; Pailhès, S.; Marques, M.A.L. Superconductivity in layered binary silicides: A density functional theory study. Phys. Rev. B 2011, 84, 184503. [Google Scholar] [CrossRef]
- Schwarz, U.; Wosylus, A.; Rosner, H.; Schnelle, W.; Ormeci, A.; Meier, K.; Baranov, A.; Nicklas, M.; Leipe, S.; Müller, C.J.; et al. Dumbbells of Five-Connected Silicon Atoms and Superconductivity in the Binary Silicides MSi3 (M = Ca, Y, Lu). J. Am. Chem. Soc. 2012, 134, 13558–13561. [Google Scholar] [CrossRef]
- Meier, K.; Cardoso-Gil, R.; Schwarz, U. Crystal structure of holmium trisilicide, HoSi3. Z. Kristallogr. 2011, 226, 297–298. [Google Scholar] [CrossRef]
- Wosylus, A.; Prots, Y.; Schwarz, U. Crystal structure of ytterbium trisilicide, YbSi3. Z. Kristallogr. NCS 2011, 226, 295–296. [Google Scholar] [CrossRef]
- Schnelle, W.; Ormeci, A.; Wosylus, A.; Meier, K.; Grin, Y.; Schwarz, U. Dumbbells of Five-Connected Ge Atoms and Superconductivity in CaGe3. Inorg. Chem. 2012, 51, 5509–5511. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Fukuoka, H.; Inumaru, K. High-Pressure Synthesis and Electronic Structure of a New Superconducting Strontium Germanide (SrGe3) Containing Ge2 Dumbbells. Inorg. Chem. 2015, 54, 7433–7437. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Schnelle, R.; Baranov, A.I.; Burkhardt, U.; Bobnar, M.; Cardoso-Gil, R.; Schwarz, U.; Grin, Y. Trigermanides AEGe3 (AE = Ca, Sr, Ba): Chemical bonding and superconductivity. Z. Naturforsch. B 2016, 71, 585–592. [Google Scholar] [CrossRef]
- Castillo, R.; Baranov, A.; Burkhardt, U.; Cardoso-Gil, R.; Schnelle, W.; Bobnar, M.; Schwarz, U. Germanium Dumbbells in a New Superconducting Modification of BaGe3. Inorg. Chem. 2016, 55, 4498–4503. [Google Scholar] [CrossRef]
- Fukuoka, H.; Tomomitsu, Y.; Inumaru, K. High-Pressure Synthesis and Superconductivity of a New Binary Barium Germanide BaGe3. Inorg. Chem. 2011, 50, 6372–6377. [Google Scholar] [CrossRef]
- Belyavina, N.M.; Markiv, V.Y.; Speka, M.V. Crystal structure of YGe3, YGe1.9 and a novel Y3Ge4 compound. J. Alloys Compd. 1999, 283, 162–168. [Google Scholar] [CrossRef]
- Fukuoka, H.; Suekuni, K.; Onimaru, T.; Inumaru, K. High-Pressure Synthesis and Superconductivity of a New Binary Lanthanum Germanide LaGe3 with Triangular Ge3 Cluster Units. Inorg. Chem. 2011, 50, 3901–3906. [Google Scholar] [CrossRef]
- Fukuoka, H.; Yamanaka, S. High-Pressure Synthesis and Properties of a Cerium Germanide CeGe3 with the Cubic Cu3Au Type Structure. Chem. Lett. 2004, 33, 1334–1335. [Google Scholar] [CrossRef]
- Castillo, R.; Baranov, A.; Burkhardt, U.; Grin, Y.; Schwarz, U. Triangular Ge3 Units in a New Modification of EuGe3. Z. Anorg. Allg. Chem. 2015, 641, 355–361. [Google Scholar] [CrossRef]
- Savysyuk, I.A.; Gladyshevskii, E.I.; Gladyshevskii, R.E. Crystal Structures of RGe3 and RGe2 Compounds (R = Y, Gd, Tb). In Proceedings of the 7th International Conference on Crystal Chemistry Intermetallic Compounds, Lviv, Ukraine, 25–28 September 1999; p. PB17. [Google Scholar]
- Schobinger-Papamantellos, P.; Rodriguez Carvajal, J.; Buschow, K.H.J. The multiple q-vector incommensurate magnetic structure of TbGe3. J. Phys. Condens. Matter 2007, 19, 236201. [Google Scholar] [CrossRef]
- Schobinger-Papamantellos, P.; de Mooij, D.B.; Buschow, K.H.J. Crystal Structures of the Compound DyGe3. J. Alloys Compd. 1992, 183, 181–186. [Google Scholar] [CrossRef]
- Schobinger-Papamantellos, P.; Rodriguez Carvajal, J.; Tung, L.D.; Ritter, C.; Buschow, K.H.J. Competing multiple-q magnetic structures in HoGe3: I. The magnetic phase diagram of HoGe3. J. Phys. Condens. Matter 2008, 20, 195201. [Google Scholar] [CrossRef]
- Eremenko, V.N.; Obushenko, I.M. Phase Diagram of the Erbium-Germanium System. Sov. Non-Ferrous Met. Res. 1981, 9, 216–220. [Google Scholar]
- Fukuoka, H.; Yoshikawa, M.; Baba, K.; Yamanaka, S. Preparation and Structures of Lanthanoid Germanides, PrGe3.36, NdGe3.25, and TmGe3 with Double Square Ge-Mesh Structures. Bull. Chem. Soc. Jpn. 2010, 83, 323–327. [Google Scholar] [CrossRef]
- Harada, M.; Fukuoka, H.; Matsumura, D.; Inumaru, K. Structure and Chemical Bonding of Binary Ytterbium Germanides, Yb3Ge5 and YbGe3, Prepared by High-Pressure and High-Temperature Reactions. J. Phys. Chem. C 2012, 116, 2153–2158. [Google Scholar] [CrossRef]
- Hübner, J.-M.; Bobnar, M.; Akselrud, L.; Prots, Y.; Grin, Y.; Schwarz, U. Lutetium Trigermanide LuGe3: High-Pressure Synthesis, Superconductivity, and Chemical Bonding. Inorg. Chem. 2018, 57, 10295–10302. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Carpenter, M.A.; Hitch, C.M. Some simplifications to multianvil devices for high pressure experiments. Am. Mineral. 1990, 75, 1020–1028. [Google Scholar]
- Young, D.A. Phase Diagrams of the Elements; UC Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Akselrud, L.; Grin, Y. WinCSD: software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Jepsen, O.; Burkhardt, A.; Andersen, O.K. The Program TB-LMTO-ASA. Version 4.7; Max-Planck-Institut für Festkörperforschung: Stuttgart, Germany, 1999. [Google Scholar]
- Von Barth, U.; Hedin, L. A local exchange-correlation potential for the spin polarized case: I. J. Phys. 1972, C5, 1629–1642. [Google Scholar] [CrossRef]
- Andersen, O.K. Linear methods in band theory. Phys. Rev. 1975, B12, 3060–3083. [Google Scholar] [CrossRef]
- Kohout, M. A measure of electron localizability. Int. J. Quantum Chem. 2004, 97, 651–658. [Google Scholar] [CrossRef]
- Kohout, M. Bonding indicators from electron pair density functionals. Faraday Discuss. 2007, 135, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kohout, M. DGrid, Versions 4.6-5.0; Radebeul, Germany, 2018. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Wagner, F.R.; Bezugly, V.; Kohout, M.; Grin, Y. Charge decomposition analysis of the Electron Localizability Indicator: A bridge between the orbital and direct representation of the chemical bond. Chem. Eur. J. 2007, 13, 5724–5741. [Google Scholar] [CrossRef] [PubMed]
- Kohout, M.; Savin, A. Atomic shell structure and electron numbers. Int. J. Quantum Chem. 1996, 60, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Grin, Y. Comprehensive Inorganic Chemistry II; Elsevier: Oxford, UK, 2013; Volume 2, pp. 359–373. [Google Scholar]
- Bende, D.; Wagner, F.R.; Grin, Y. 8—N Rule and Chemical Bonding in Main-Group MgAgAs-Type Compounds. Inorg. Chem. 2015, 54, 3970–3978. [Google Scholar] [CrossRef]
- Evers, J. Transformation of Three-Connected Silicon in BaSi2. J. Solid State Chem. 1980, 32, 77–86. [Google Scholar] [CrossRef]
- Goebel, T.; Prots, Y.; Haarmann, F. Refinement of the crystal structure of dibarium tetrasilicide, Ba2Si4. Z. Kristallogr. NCS 2009, 224, 7–8. [Google Scholar] [CrossRef]
- Dutta, B.N. Lattice constants and thermal expansion of silicon up to 900 °C by X-ray method. Phys. Status Solidi B 1962, 2, 984–987. [Google Scholar] [CrossRef]
- Obinata, I.; Takeuchi, Y.; Kurihara, K.; Watanabe, W. Über die Legierungen des Mangans und Siliziums mit Alkali- und Erdalkalimetallen. Metallurgy 1965, 19, 21–35. [Google Scholar]
- Shi, J.; Cui, W.; Flores-Livas, J.A.; San-Miguel, A.; Botti, S.; Marques, M.A.L. Investigation of new phases in the Ba–Si phase diagram under high pressure using ab initio structural search. Phys. Chem. Chem. Phys. 2016, 18, 8108–8114. [Google Scholar] [CrossRef]
- Yamanaka, S.; Maekawa, S. Structural Evolution of the Binary System Ba-Si under High-pressure and High-temperature Conditions. Z. Naturforsch. B 2006, 61, 1493–1499. [Google Scholar] [CrossRef]
- Janzon, K.H.; Schäfer, H.; Weiss, A. Zur Kenntnis der Disilicide der Erdalkalimetalle. Z. Anorg. Allg. Chem. 1970, 372, 87–99. [Google Scholar] [CrossRef]
- Evers, J.; Oehlinger, G.; Weiss, A. Kristallstruktur von Bariumdisilicid bei hohen Drücken. Angew. Chem. 1977, 89, 673–674. [Google Scholar] [CrossRef]
- Evers, J.; Oehlinger, G.; Weiss, A. Eine neue Hochdruckphase von Bariumdisilicid. Angew. Chem. 1978, 90, 562–563. [Google Scholar] [CrossRef]
- The following idealized atomic positions have been used for the band structure computations in order to eliminate the effects of disorder: Ba1, 4e (0, 0, 0.17865); Ba2, 4d (1/2, 0, 1/4); Si1, 8f (0.3468, 0, 0); Si2, 8i (0.3324, x, 0.1014); Si3, 8i (0.2184, x, 0.4221).
- Wosylus, A.; Rosner, H.; Schnelle, W.; Schwarz, U.Z. Crystal Structure Refinement and Electronic Properties of Si(cI16). Z. Anorg. Allg. Chem. 2009, 635, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Yim, W.M.; Paff, R.J. Thermal expansion of AlN, sapphire, and silicon. J. Appl. Phys. 1974, 45, 1456–1457. [Google Scholar] [CrossRef]
- Ormeci, A.; Grin, Y. Coexistence of ionic and covalent atomic interactions (bonding inhomogeneity) and thermoelectric properties of intermetallic clathrates. J. Thermoelectr. 2015, 6, 16–32. [Google Scholar]
- Grin, Y.; Fedorchuk, A.; Faria, R.J.; Wagner, F.R. Atomic Charges and Chemical Bonding in Y-Ga Compounds. Crystals 2018, 8, 99. [Google Scholar] [CrossRef]
- Kohout, M.; Wagner, F.R.; Grin, Y. Electron localization function for transition-metal compounds. Theor. Chem. Acc. 2002, 108, 150–156. [Google Scholar] [CrossRef]
- Grin, Y.; Wedig, U.; von Schnering, H.G. Hyperbolische Elektronenpaar-Struktur in RhBi4. Angew. Chem. Int. Ed. Engl. 1995, 34, 1204–1206. [Google Scholar] [CrossRef]
- Schwarz, U.; Tencé, S.; Janson, O.; Koz, C.; Krellner, C.; Burkhardt, U.; Rosner, H.; Steglich, F.; Grin, Y. CoBi3: A Binary Cobalt-Bismuth Compound and Superconductor. Angew. Chem. Int. Ed. 2013, 52, 9853–9857. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Akselrud, L.; Bobnar, M.; Burkhardt, U.; Schmidt, M.; Zhao, J.-T.; Schwarz, U.; Grin, Y. Weak Interactions under Pressure: Hp-CuBi and Its Analogues. Angew. Chem. Int. Ed. 2017, 56, 5620–5624. [Google Scholar] [CrossRef]
- Chow, T.P.; Steckl, A.J. Refractory metal silicides: Thin-film properties and processing technology. IEEE Trans. Electron Devices 1983, 30, 1480–1497. [Google Scholar] [CrossRef]
- Flores-Livas, J.A.; Debord, R.; Botti, S.; San Miguel, A.; Marques, M.A.L.; Pailhes, S. Enhancing the Superconducting Transition Temperature of BaSi2 by Structural Tuning. Phys. Rev. Lett. 2011, 106, 087002. [Google Scholar] [CrossRef] [PubMed]








| Composition | BaSi3 |
|---|---|
| Space group, Pearson symbol | I2m (no. 121), tI32 |
| Structure type | BaSi3 |
| Unit cell parameters | |
| a/Å | 7.6971(2) |
| c/Å | 12.6605(3) |
| V/Å3 | 750.07(5) |
| Formula units per unit cell, Z | 4 |
| Measurement range | 1.0° ≤ 2θ ≤ 29.0°, 0.002° step width |
| Measured points/reflections | 14000/281 |
| R(P)/R(wP) | 0.073/0.099 |
| Atom | Site | SOF | x/a | y/b | z/c | Biso |
|---|---|---|---|---|---|---|
| Ba1 | 8i | 0.5 | 0.0162(1) | x | 0.1785(2) | 0.67(2) |
| Ba2 | 4d | 1.0 | 1/2 | 0 | 1/4 | 0.60(2) |
| Si1 | 8f | 1.0 | 0.347(1) | 0 | 0 | 1.37(2) |
| Si2 | 8i | 1.0 | 0.332(3) | x | 0.1012(9) | 1.16(2) |
| Si3 | 16j | 0.5 | 0.2078(9) | 0.2282(9) | 0.422(1) | 1.24(2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübner, J.-M.; Akselrud, L.; Schnelle, W.; Burkhardt, U.; Bobnar, M.; Prots, Y.; Grin, Y.; Schwarz, U. High-Pressure Synthesis and Chemical Bonding of Barium Trisilicide BaSi3. Materials 2019, 12, 145. https://doi.org/10.3390/ma12010145
Hübner J-M, Akselrud L, Schnelle W, Burkhardt U, Bobnar M, Prots Y, Grin Y, Schwarz U. High-Pressure Synthesis and Chemical Bonding of Barium Trisilicide BaSi3. Materials. 2019; 12(1):145. https://doi.org/10.3390/ma12010145
Chicago/Turabian StyleHübner, Julia-Maria, Lev Akselrud, Walter Schnelle, Ulrich Burkhardt, Matej Bobnar, Yurii Prots, Yuri Grin, and Ulrich Schwarz. 2019. "High-Pressure Synthesis and Chemical Bonding of Barium Trisilicide BaSi3" Materials 12, no. 1: 145. https://doi.org/10.3390/ma12010145