Abstract
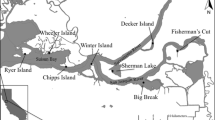
Die-back of Phragmites australis in the Mississippi River Delta (MRD), Louisiana, USA, is associated with large populations of nonnative Roseau Cane Scale (RCS), Nipponaclerda biwakoensis. Initial observations suggested different P. australis phenotypes displayed different susceptibilities to scale infestation and die-back, but the role of scale infestation on die-back was unknown. To understand the effect of RCS on P. australis, paired stands of Delta and European phenotypes in the MRD were monitored for stem heights, densities, and scale infestation over two years. A mesocosm experiment was conducted to assess whether RCS abundance and P. australis growth were dependent on water salinity and phenotype. Three Phragmites australis phenotypes were grown in small pools under fresh or mesohaline conditions, and RCS infestated or non-infested treatments. Scale densities were up to 7 times greater on the Delta compared to the European phenotype. Scale infestation resulted in 22%–39% reductions in the proportion of stems with green leaf tissue for all phenotypes, and 12% reduction in stem heights for Delta-type. Salinity was detrimental to all phenotypes, reducing stem heights by 20% compared to freshwater. Our results provide evidence that the RCS can result in die-back symptoms similar to what is observed in the MRD.


Similar content being viewed by others

References
Achenbach L, Brix H (2014) Can differences in salinity tolerance explain the distribution of four genetically distinct lineages of Phragmites australis in the Mississippi River Delta? Hydrobiologia 737:5–23
Allen WJ, Meyerson LA, Cummings D, Anderson J, Bhattarai GP, Cronin JT (2017) Biogeography of a plant invasion: drivers of latitudinal variation in enemy release. Global Ecology and Biogeography 26:435–446
Armstrong J, Armstrong W, Armstrong I et al (1996a) Senescence, and phytotoxin, insect, fungal and mechanical damage: factors reducing convective gas-flows in Phragmites australis. Aquatic Botany 54:211–226
Armstrong J, Armstrong W, Wd P (1996b) Phragmites die–back: bud and root death, blockages within the aeration and vascular systems and the possible role of phytotoxins. New Phytologist 133:399–414
Bhattarai GP, Meyerson LA, Cronin JT (2017) Geographic variation in apparent competition between native and invasive Phragmites australis. Ecology 98:349–358
Blossey B, Häfliger P, Tewksbury P et al (2018) Host specificity and risk assessment of Archanara geminipuncta and Archanara neurica, two potential biocontrol agents for invasive Phragmites australis in North America. Biological Control 125:98–112
Bowling RD, Brewer MJ, Kerns DL, et al. (2016) Sugarcane aphid (Hemiptera: Aphididae): a new pest on sorghum in North America. Journal of Integrated Pest Management 7:12. https://doi.org/10.1093/jipm/pmw011
Brix H, Ye S, Laws EA, Sun D, Li G, Ding X, Yuan H, Zhao G, Wang J, Pei S (2014) Large-scale management of common reed, Phragmites australis, for paper production: a case study from the Liaohe Delta, China. Ecological Engineering 73:760–769
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64:261–273
Coastal Protection and Restoration Authority (CPRA) of Louisiana (2019) Coastwide Reference Monitoring System-Wetlands Monitoring Data. Retrieved from Coastal Information Management System (CIMS) database. http://cims.coastal.louisiana.gov. Accessed 14 June 2019
Cronin JT, Bhattarai GP, Allen WJ, Meyerson LA (2015) Biogeography of a plant invasion: plant–herbivore interactions. Ecology 96:1115–1127
Day JW Jr, Barras J, Clairain E, Johnston J, Justic D, Kemp GP, Ko JY, Lane R, Mitsch WJ, Steyer G, Templet P, Yañez-Arancibia A (2005) Implications of global climate change and energy cost and availability for the restoration of the Mississippi delta. Ecological Engineering 24:253–365
Eichele-Nelson JL, Wick AF, DeSutter TM et al (2017) The effects of salinity on the herbivorous crop pest Tetranychus urticae (Trombidiformes: Tetranychidae) on soybean and corn. Environmental Entomology 46:839–846
Eller F, Lambertini C, Nguyen LX, Brix H (2014) Increased invasive potential of non-native Phragmites australis: elevated CO2 and temperature alleviate salinity effects on photosynthesis and growth. Global Change Biology 20:531–543
Games PA, Howell JF (1976) Pairwise multiple comparison procedures with unequal n’s and/or variances: a Monte Carlo study. Journal of Educational Statistics 1:113–125
Hauber DP, Saltonstall K, White DA, Hood CS (2011) Genetic variation in the common reed, Phragmites australis, in the Mississippi River Delta marshes: evidence for multiple introductions. Estuaries and Coasts 34:851–862
Hazelton EL, Mozdzer TJ, Burdick DM, et al. (2014) Phragmites australis management in the United States: 40 years of methods and outcomes. AoB plants 6. https://doi.org/10.1093/aobpla/plu001
Hellings SE, Gallagher JL (1992) The effects of salinity and flooding on Phragmites australis. Journal of Applied Ecology 29:41–49
Hodges G, Hodges A (2004) New invasive species of mealybugs, Palmicultor lumpurensis and Chaetococcus bambusae (Hemiptera: Coccoidea: Pseudococcidae), on bamboo in Florida. Florida Entomologist 87:396–397
Howard RJ, Rafferty PS (2006) Clonal variation in response to salinity and flooding stress in four marsh macrophytes of the northern Gulf of Mexico, USA. Environmental and Experimental Botany 56:301–313
Kaneko S (2004) Within-plant vertical distributions of the scale insect Nipponaclerda biwakoensis and its five parasitoids that exhibit frequent successful multiparasitism on the common reed. Entomological Science 7:331–339
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Kiviat E (2013) Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB Plants 5:plt008
Knight IA, Wilson BE, Gill M et al (2018) Invasion of Nipponaclerda biwakoensis (Hemiptera: Aclerdidae) and Phragmites australis die-back in southern Louisiana, USA. Biological Invasions 20: 2739–2744
Kuwana S (1907) Coccidae of Japan, I. a synoptical list of Coccidae of Japan with descriptions of thirteen new species. Bulletin of the Imperial Central Agricultural Experiment Station, Japan 1:177–212
Lambert AM, Casagrande RA (2007) Susceptibility of native and non-native common reed to the non-native mealy plum aphid (Homoptera: Aphididae) in North America. Environmental Entomology 36:451–457
Lambertini C, Mendelssohn IA, Gustafsson MH et al (2012) Tracing the origin of Gulf Coast Phragmites (Poaceae): a story of long-distance dispersal and hybridization. American Journal of Botany 99:538–551
League MT, Colbert EP, Seliskar DM, Gallagher JL (2006) Rhizome growth dynamics of native and exotic haplotypes of Phragmites australis (common reed). Estuaries and Coasts 29:269–276
Lissner J, Schierup H-H (1997) Effects of salinity on the growth of Phragmites australis. Aquatic Botany 55:247–260
McDonald ME (1955) Cause and effects of a die-off of emergent vegetation. The Journal of Wildlife Management 19:24–35
Meyerson LA, Lambert AM, Saltonstall K (2010) A tale of three lineages: expansion of common reed (Phragmites australis) in the U.S. southwest and Gulf Coast. Invasive Plant Science and Management 3:515–520
Mozdzer TJ, Zieman JC (2010) Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in north American Atlantic coast wetlands. Journal of Ecology 98:451–458
Mozdzer TJ, Brisson J, Hazelton EL (2013) Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB Plants 5. https://doi.org/10.1093/aobpla/plt048
Natural Resources Conservation Service (NRCS), United States Department of Agriculture (2019) Web Soil Survey. https://websoilsurvey.sc.egov.usda.gov/. Accessed 14 June 2019
Park MG, Blossey B (2008) Importance of plant traits and herbivory for invasiveness of Phragmites australis (Poaceae). American Journal of Botany 95:1557–1568
Price AL, Fant JB, Larkin DJ (2014) Ecology of native vs. introduced Phragmites australis (common reed) in Chicago-area wetlands. Wetlands 34:369–377
Qin C, Sun J, Jing X et al (2003) Preliminary report on ecological control techniques of reed pests in salted sea breaches. Plant Protection Technology and Extension 2:22–24 (in Chinese with English abstract)
Ramsey EW, III, Rangoonwala A. (2017) Mapping the change of Phragmites australis live biomass in the lower Mississippi River Delta marshes: U.S. Geological Survey Open-File Report 2017–1098, https://doi.org/10.3133/ofr20171098
Renault S, Wolfe S, Markham J, et al. (2016) Increased resistance to a generalist herbivore in a salinity-stressed non-halophytic plant. AoB Plants 8. https://doi.org/10.1093/aobpla/plw028
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences 99:2445–2449
Saltonstall K, Stevenson JC (2007) The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquatic Botany 86:331–336
Sun H, Brown A, Coppen J, Steblein P (2007) Response of Phragmites to environmental parameters associated with treatments. Wetlands Ecology and Management 15:63–79
Tachikawa T (1970) Notes on some Japanese species of Encyrtidae (Hymenoptera: Chalcidoidea). Transactions of the Shikoku Entomological Society 10:100–103
Thaler JS, Bostock RM (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85:48–58
van der Putten WH (1997) Die-back of Phragmites australis in European wetlands: an overview of the European research programme on reed die-back and progression (1993–1994). Aquatic Botany 59:263–275
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG (2005) Salt tolerance underlies the cryptic invasion of north American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Marine Ecology Progress Series 298:1–8
Wang Y, Mopper S, Hasenstein KH (2001) Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. Journal of Chemical Ecology 27:327–342
Acknowledgements
This project was funded in part by the Louisiana State University AgCenter, Louisiana Department of Wildlife and Fisheries, Louisiana Department of Agriculture and Forestry, Louisiana Coastal Protection and Restoration Authority, Coastal Wetlands Planning, Protection and Restoration Act and NSF grant DMS-1516833 (to J.T.C.). This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number LAB94095. We thank Todd Baker, Vaughan McDonald, Trebor Victoriano and other LDWF personnel for technical and logistical support. We also thank Jeremy Rodriguez and Joey Breaux (LADAF) for support during field collections. Lastly we recognize the contributions of the graduate and undergraduate students of the Cronin, Diaz, and Wilson labs at Louisiana State University for assistance in processing of samples. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Knight, I.A., Cronin, J.T., Gill, M. et al. Investigating Plant Phenotype, Salinity, and Infestation by the Roseau Cane Scale as Factors in the Die-Back of Phragmites australis in the Mississippi River Delta, USA. Wetlands 40, 1327–1337 (2020). https://doi.org/10.1007/s13157-020-01307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-020-01307-3