Abstract
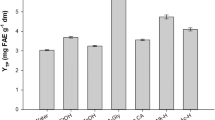
Potato peel is a major underutilized by-product stream from potato processing industry and a potential source of valuable bioactives such as antioxidants. A Sequential Hydrothermal Extraction (SeqHTE) process was employed for recovering compounds of high commercial value from the peels. Process performance was evaluated in terms of effectiveness, quality and yields of the bioactive extracts. The highest recoveries of polyphenols were 22.48 and 32.87 mg/g dry peel from the Russet Burbank and peel mixture sample, respectively. The extracts displayed significant antioxidant activities, measured as free radical inhibition, ranging from 40 to 92%. Moreover, glycoalkaloids, polysaccharides, and soluble nutrients were also recovered through the SeqHTE process. Alkaloid extraction ranged from 20 to 450 and from 35 to 610 mg/kg dry peel for the Russet Burbank and peel mixture, respectively. Similarly, polysaccharide yield varied from 0 to 35.7 wt%. Separating these compounds significantly reduced solid content in the remaining stream, which may effectively alleviate concerns about adverse environmental impacts and costs associated with handling raw potato peels. These results demonstrated the suitability of SeqHTE as a platform for valorizing waste biomass by fractionating and recovering high value compounds from it.
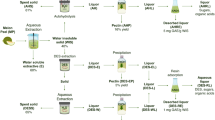
Graphic Abstract






Similar content being viewed by others
References
Liang, S., McDonald, A.G., Coats, E.R.: Lactic acid production with undefined mixed culture fermentation of potato peel waste. Waste Manag. 34(11), 2022–2027 (2014)
Schieber, A., Saldaña, M.D.A.: Potato peels: a source of nutritionally and pharmacologically interesting compounds-a review. Food. 3(2), 23–29 (2009)
Amado, I.R., Franco, D., Sánchez, M., Zapata, C., Vázquez, J.A.: Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 165, 290–299 (2014)
Al-Weshahy, A., Rao, V.A.: Potato peel as a source of important phytochemical antioxidant nutraceuticals and their role in human health-a review. INTECH Open Access Publisher, London (2012)
Banerjee, J., Singh, R., Vijayaraghavan, R., MacFarlane, D., Patti, A.F., Arora, A.: Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 225, 10–22 (2017)
Hossain, M.B., Tiwari, B.K., Gangopadhyay, N., O’Donnell, C.P., Brunton, N.P., Rai, D.K., et al.: Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 21(4), 1470–1476 (2014)
Friedman, M., Huang, V., Quiambao, Q., Noritake, S., Liu, J., Kwon, O., et al.: Potato peels and their bioactive glycoalkaloids and phenolic compounds inhibit the growth of pathogenic trichomonads. J. Agric. Food Chem. 66(30), 7942–7947 (2018)
Wijngaard, H.H., Ballay, M., Brunton, N.: The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 133(4), 1123–1130 (2012)
Jeddou, K.B., Chaari, F., Maktouf, S., Nouri-Ellouz, O., Helbert, C.B., Ghorbel, R.E.: Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 205, 97–105 (2016)
Wu, D.: Recycle technology for potato peel waste processing: a review. Procedia Environ. Sci. 31, 103–107 (2016)
Schieber, A., Stintzing, F.C., Carle, R.: By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci. Technol. 12(11), 401–413 (2001)
Escarpa, A., González, M.C.: Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 427(1), 119–127 (2001)
Kosseva, M.R.: Processing of food wastes. Adv. Food Nutr. Res. 58, 57–136 (2009)
Herrero, M., Cifuentes, A., Ibañez, E.: Sub-and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem. 98(1), 136–148 (2006)
King, J.W., Grabiel, R.D.: Isolation of polyphenolic compounds from fruits or vegetables utilizing sub-critical water extraction. US Patent 7,208,181. USDA (2007)
Singh, P.P., Saldaña, M.D.A.: Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 44(8), 2452–2458 (2011)
Duba, K.S., Fiori, L.: Extraction of bioactives from food processing residues using techniques performed at high pressures. Curr. Opin. Food Sci. 5, 14–22 (2015)
Miao, C., Chakraborty, M., Dong, T., Yu, X., Chi, Z., Chen, S.: Sequential hydrothermal fractionation of yeast Cryptococcus curvatus biomass. Bioresour. Technol. 164, 106–112 (2014)
de Araújo Padilha, C.E., da Costa, N.C., Oliveira Filho, M.A., de Sousa Júnior, F.C., de Assis, C.F., de Santana Souza, D.F., et al.: Fractionation of green coconut fiber using sequential hydrothermal/alkaline pretreatments and Amberlite XAD-7HP resin. J. Environ. Chem. Eng. 7(6), 103474 (2019)
Toor, S.S., Rosendahl, L., Rudolf, A.: Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy. 36(5), 2328–2342 (2011)
Savage, P.E.: Organic chemical reactions in supercritical water. Chem. Rev. 99(2), 603–622 (1999)
Brunner, G.: Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 47(3), 373–381 (2009)
Akiya, N., Savage, P.E.: Roles of water for chemical reactions in high-temperature water. Chem. Rev. 102(8), 2725–2750 (2002). https://doi.org/10.1021/cr000668w
Libra, J.A., Ro, K.S., Kammann, C., Funke, A., Berge, N.D., Neubauer, Y., et al.: Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1), 71–106 (2011)
Martinez-Fernandez, J.S., Chen, S.: Sequential hydrothermal liquefaction characterization and nutrient recovery assessment. Algal Res. 25, 274 (2017)
Friedman, M., Roitman, J.N., Kozukue, N.: Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem. 51(10), 2964–2973 (2003)
Jarén, C., López, A., Arazuri, S.: Advanced analytical techniques for quality evaluation of potato and its products. In: Singh, J., Kaur, L. (eds.) Advances in potato chemistry and technology, 2nd edn, pp. 563–602. Elsevier, Amsterdam (2016)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., et al.: Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 1617, 1–16 (2008)
Ko, M.-J., Cheigh, C.-I., Chung, M.-S.: Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 143, 147–155 (2014)
Chakraborty, M., McDonald, A.G., Nindo, C., Chen, S.: An α-glucan isolated as a co-product of biofuel by hydrothermal liquefaction of Chlorella sorokiniana biomass. Algal Res. 2(3), 230–236 (2013)
Bouchard, A., Hofland, G.W., Witkamp, G.-J.: Properties of sugar, polyol, and polysaccharide water—ethanol solutions. J. Chem. Eng. Data 52(5), 1838–1842 (2007)
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3), 144–158 (1965)
Waterhouse, A.L.: Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 6, l1 (2002)
Berry, J.H.J.: UHPLC of Polyphenols in Red Wine. https://www.agilent.com/cs/library/applications/: Agilent Technologies (2010). Accessed 19 Sept 2018
Friedman, M.: Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 1054(1), 143–155 (2004)
Brand-Williams, W., Cuvelier, M.-E., Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28(1), 25–30 (1995)
Sant’ Anna, V., Brandelli, A., Marczak, L.D.F., Tessaro, I.C.: Kinetic modeling of total polyphenol extraction from grape marc and characterization of the extracts. Sep. Purif. Technol. 100, 82–87 (2012)
Pinelo, M., Rubilar, M., Jerez, M., Sineiro, J., Núñez, M.J.: Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 53(6), 2111–2117 (2005)
Gai, C., Zhang, Y., Chen, W.-T., Zhou, Y., Schideman, L., Zhang, P., et al.: Characterization of aqueous phase from the hydrothermal liquefaction of Chlorella pyrenoidosa. Bioresour. Technol. 184, 328–335 (2015)
Gao, Y., Chen, H., Wang, J., Shi, T., Yang, H.-P., Wang, X.-H.: Characterization of products from hydrothermal liquefaction and carbonation of biomass model compounds and real biomass. J. Fuel Chem. Technol. 39(12), 893–900 (2011)
Zha, S., Zhao, Q., Chen, J., Wang, L., Zhang, G., Zhang, H., et al.: Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii). Carbohydr. Polym. 111, 584–587 (2014)
Navarre, D.A., Shakya, R., Hellmann, H.: Vitamins, phytonutrients, and minerals in potato. In: Singh, J., Kaur, L. (eds.) Advances in potato chemistry and technology, 2nd edn, pp. 117–166. Elsevier, Amsterdam (2016)
Liang, S., McDonald, A.G.: Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 62(33), 8421–8429 (2014)
Önal, E.P., Uzun, B.B., Pütün, A.E.: Steam pyrolysis of an industrial waste for bio-oil production. Fuel Process. Technol. 92(5), 879–885 (2011)
Farvin, K.H.S., Grejsen, H.D., Jacobsen, C.: Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): effect on lipid and protein oxidation. Food Chem. 131(3), 843–851 (2012)
Vamvuka, D., Pitharoulis, M., Alevizos, G., Repouskou, E., Pentari, D.: Ash effects during combustion of lignite/biomass blends in fluidized bed. Renew. Energy. 34(12), 2662–2671 (2009)
Li, C., Aston, J.E., Lacey, J.A., Thompson, V.S., Thompson, D.N.: Impact of feedstock quality and variation on biochemical and thermochemical conversion. Renew. Sustain. Energy Rev. 65, 525–536 (2016)
Alvarez, V.H., Cahyadi, J., Xu, D., Saldaña, M.D.A.: Optimization of phytochemicals production from potato peel using subcritical water: experimental and dynamic modeling. J. Supercrit. Fluids. 90, 8–17 (2014)
Tsao, R.: Chemistry and biochemistry of dietary polyphenols. Nutrients. 2(12), 1231–1246 (2010)
Cvetanović, A., Švarc-Gajić, J., Zeković, Z., Jerković, J., Zengin, G., Gašić, U., et al.: The influence of the extraction temperature on polyphenolic profiles and bioactivity of chamomile (Matricaria chamomilla L.) subcritical water extracts. Food Chem. 271, 328–337 (2019)
Cocero Alonso, M.J., Abad Fernández, N., Adamovic, T., Vaquerizo Martín, L., Martínez Fajardo, C., Pazo Cepeda, M.V.: Understanding biomass fractionation in subcritical & supercritical water. J. Supercrit. Fluids (2017). https://doi.org/10.1016/j.supflu.2017.08.012
Teo, C.C., Tan, S.N., Yong, J.W.H., Hew, C.S., Ong, E.S.: Pressurized hot water extraction (PHWE). J. Chromatogr. A. 1217(16), 2484–2494 (2010)
Carr, A.G., Mammucari, R., Foster, N.R.: A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 172(1), 1–17 (2011)
Srinivas, K., King, J.W., Howard, L.R., Monrad, J.K.: Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 100(2), 208–218 (2010)
Faeth, J.L., Valdez, P.J., Savage, P.E.: Fast hydrothermal liquefaction of Nannochloropsis sp. to produce biocrude. Energy Fuels. 27(3), 1391–1398 (2013)
Jaromír, L., Karel, H., Matyáš, O.: Colored potatoes. In: Singh, J., Kaur, L. (eds.) Advances in potato chemistry and technology, 2nd edn, pp. 249–281. Elsevier, Amsterdam (2016)
Balasundram, N., Sundram, K., Samman, S.: Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 99(1), 191–203 (2006)
Al-Weshahy, A., Rao, A.V.: Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Res. Int. 42(8), 1062–1066 (2009)
Deußer, H., Guignard, C., Hoffmann, L., Evers, D.: Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 135(4), 2814–2824 (2012)
Singh, A., Sabally, K., Kubow, S., Donnelly, D.J., Gariepy, Y., Orsat, V., et al.: Microwave-assisted extraction of phenolic antioxidants from potato peels. Molecules 16(3), 2218–2232 (2011)
Moure, A., Cruz, J.M., Franco, D., Domınguez, J.M., Sineiro, J., Domınguez, H., et al.: Natural antioxidants from residual sources. Food Chem. 72(2), 145–171 (2001)
Naczk, M., Shahidi, F.: Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 41(5), 1523–1542 (2006)
Nara, K., Miyoshi, T., Honma, T., Koga, H.: Antioxidative activity of bound-form phenolics in potato peel. Biosci. Biotechnol. Biochem. 70(6), 1489–1491 (2006)
Sato, Y., Itagaki, S., Kurokawa, T., Ogura, J., Kobayashi, M., Hirano, T., et al.: In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 403(1), 136–138 (2011)
Lafka, T.-I., Sinanoglou, V., Lazos, E.S.: On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 104(3), 1206–1214 (2007)
Ciriminna, R., Carnaroglio, D., Delisi, R., Arvati, S., Tamburino, A., Pagliaro, M.: Industrial feasibility of natural products extraction with microwave technology. Chem. Select. 1(3), 549–555 (2016)
Waglay, A., Karboune, S.: Potato proteins functional food ingredients. In: Singh, J., Kaur, L. (eds.) Advances in potato chemistry and technology, 2nd edn, pp. 75–104. Elsevier, Amsterdam (2016)
Ben, J.K., Bouaziz, F., Helbert, C.B., Nouri-Ellouz, O., Maktouf, S., Ellouz-Chaabouni, S., et al.: Structural functional and biological properties of potato peel oligosaccharides. Int. J. Biol. Macromol. 112, 1146–1155 (2018)
Plaza, M., Amigo-Benavent, M., del Castillo, M.D., Ibáñez, E., Herrero, M.: Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 43(4), 1123–1129 (2010)
Friedman, M.: Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J. Agric. Food Chem. 54(23), 8655–8681 (2006)
Rayburn, J.R., Bantle, J.A., Friedman, M.: Role of carbohydrate side chains of potato glycoalkaloids in developmental toxicity. J. Agric. Food Chem. 42(7), 1511–1515 (1994)
Nikolic, N.C., Stankovic, M.Z.: Solanidine hydrolytic extraction and separation from the potato (Solanum tuberosum L.) vines by using solid−liquid−liquid systems. J. Agric. Food Chem. 51(7), 1845–1849 (2003)
Friedman, M., McDonald, G.M.: Acid-catalyzed partial hydrolysis of carbohydrate groups of the potato glycoalkaloid. alpha.-chaconine in alcoholic solutions. J. Agric. Food Chem. 43(6), 1501–1506 (1995)
Friedman, M., McDonald, G., Haddon, W.F.: Kinetics of acid-catalyzed hydrolysis of carbohydrate groups of potato glycoalkaloids. Alpha.-chaconine and alpha-solanine. J. Agric. Food Chem. 41(9), 1397–1406 (1993)
Peterson, A.A., Vogel, F., Lachance, R.P., Fröling, M., Antal, J.M.J., Tester, J.W.: Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ. Sci. 1(1), 32 (2008)
Rogalinski, T., Liu, K., Albrecht, T., Brunner, G.: Hydrolysis kinetics of biopolymers in subcritical water. J. Supercrit. Fluids. 46(3), 335–341 (2008)
Ben Taher, I., Fickers, P., Chniti, S., Hassouna, M.: Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol. Prog. 33, 397 (2017)
Chakraborty, M., Miao, C., McDonald, A., Chen, S.: Concomitant extraction of bio-oil and value added polysaccharides from Chlorella sorokiniana using a unique sequential hydrothermal extraction technology. Fuel 95, 63–70 (2012)
Ben, J.K., Bouaziz, F., Zouari-Ellouzi, S., Chaari, F., Ellouz-Chaabouni, S., Ellouz-Ghorbel, R., et al.: Improvement of texture and sensory properties of cakes by addition of potato peel powder with high level of dietary fiber and protein. Food Chem. 217, 668–677 (2017)
Ahamed, A., Yin, K., Ng, B.J.H., Ren, F., Chang, V.-C., Wang, J.-Y.: Life cycle assessment of the present and proposed food waste management technologies from environmental and economic impact perspectives. J. Clean. Prod. 131, 607–614 (2016)
Elliott, D.C.: Hydrothermal processing. Wiley, Chichester, UK (2011)
Lucian, M., Volpe, M., Gao, L., Piro, G., Goldfarb, J.L., Fiori, L.: Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 233, 257–268 (2018)
Lucian, M., Volpe, M., Fiori, L.: Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: experimental data and modeling. Energies. 12(3), 516 (2019)
Valdez, P.J., Nelson, M.C., Wang, H.Y., Lin, X.N., Savage, P.E.: Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass Bioenergy 46, 317–331 (2012)
Bertoft, E., Blennow, A.: Structure of potato starch. In: Singh, J., Kaur, L. (eds.) Advances in potato chemistry and technology, 2nd edn, pp. 57–73. Elsevier, Amsterdam (2016)
Cordell, D., Drangert, J.-O., White, S.: The story of phosphorus: global food security and food for thought. Glob. Environ. Chang. 19(2), 292–305 (2009)
Barreiro, D.L., Bauer, M., Hornung, U., Posten, C., Kruse, A., Prins, W.: Cultivation of microalgae with recovered nutrients after hydrothermal liquefaction. Algal Res. 9, 99–106 (2015)
Acknowledgements
This work was supported by the US Department Agriculture National Institute of Food and Agriculture Grant 2018-67021-27719. The authors thank J. R. Simplot Company for supplying the peel samples employed in this study. Moreover, the authors sincerely thank Mrs. Embrey Bronstad for her collaboration in revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinez-Fernandez, J.S., Seker, A., Davaritouchaee, M. et al. Recovering Valuable Bioactive Compounds from Potato Peels with Sequential Hydrothermal Extraction. Waste Biomass Valor 12, 1465–1481 (2021). https://doi.org/10.1007/s12649-020-01063-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01063-9