Abstract
Purpose
Octopus by-products are considered as a waste and no data of the activity of their lipid fractions have ever been reported. The aim of this study was to valorize Octopus vulgaris by-products by investigating their dichloromethane extract effect on proliferation and apoptosis of human breast cancer cell lines.
Methods
Two lipidics subfractions, F3336 and F3740, of Octopus by-products were obtained by silica gel chromatography after total lipid extraction. Their effects on proliferation, migration and apoptosis were examined on MCF-7 and MDA-MB-231 human breast cancer cell lines. The 3-(4,5-dimethyl-2-thiazol)-2,5 diphenyltetrazolium bromides (MTT) assay was used for the cell viability. Cell death was determined by flow cytometric analysis after 7-aminoactinomycin D (7-AAD) staining. MDA-MB-231 invasion and migration were analyzed using the Boyden chamber and wound healing assay.
Results
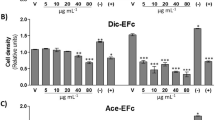
Treatment with the two lipidics subfractions showed a reduction in the proliferation of MCF-7 and MDA-MB-231 cell lines in a dose dependent manner. Treatments significantly inhibited MCF-7 cells growth than MDA-MB 231 cells. Moreover, the two lipidics subfractions induced apoptosis of MCF-7 cells and reduced invasion and migration of MDA-MB 231 cells.
Conclusion
This study shows for the first time that O. vulgaris lipid extracts have an antiproliferative and apoptotic effects on human breast cancer cell lines.





Similar content being viewed by others
References
The State of World Fisheries and Aquaculture. Food and Agriculture Organization, United Nations, Rome (2014). www.fao.org/3/a-i3720e
Chamras, H., Ardashian, A., Heber, D., Glaspy, J.A.: Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 13, 711–716 (2002)
Sakakima, Y., Hayakawa, A., Nagasaka, T., Nakao, A.: Prevention of hepatocarcinogenesis with phosphatidylcholine and menaquinone-4: in vitro and in vivo experiments. J. Hepatol. 47, 83–92 (2007)
Byju, K., Anuradha, V., Vasundhara, G., Nair, S.M., Kumar, N.C.: In vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral, subergorgia reticulata. Pharmacogn. Mag. 10, 65–71 (2014)
Mendilaharsu, M., De Stefani, E., Deneo-Pellegrini, H., Carzoglio, J., Ronco, A.: Phytosterols and risk of lung cancer: a case–control study in Uruguay. Lung Cancer 21, 37–45 (1998)
De Stefani, E., Boffetta, P., Ronco, A.L., Brennan, P., Deneo-Pellegrini, H., Carzoglio, J.C., Mendilaharsu, M.: Plant sterols and risk of stomach cancer: a case–control study in Uruguay. Nutr. Cancer 37, 140–144 (2000)
McCann, S.E., Freudenheim, J.L., Marshall, J.R., Graham, S.: Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J. Nutr. 133, 1937–1942 (2003)
Ju, Y.H., Clausen, L.M., Allred, K.F., Almada, A.L., Helferich, W.G.: β-Sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovarioectomized athymic mice. J. Nutr. 134, 1145–1151 (2004)
El Roz, A., Bard, J.M., Huvelin, J.M., Nazih, H.: LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 32, 3007–3013 (2012)
El Roz, A., Bard, J.M., Huvelin, J.M., Nazih, H.: The anti-proliferative and pro-apoptotic effects of the trans9, trans11 conjugated linoleic acid isomer on MCF-7 breast cancer cells are associated with LXR activation. Prostaglandins Leukot. Essent. Fat. Acids 88, 265–272 (2012)
Lu, X., Liu, J., Hou, F., Liu, Z., Cao, X., Seo, H., Gao, B.: Cholesterol induces pancreatic β cell apoptosis through oxidative stress pathway. Cell Stress Chaperones 16, 539–548 (2011)
Folch, J., Lees, M., Sloane-Stanley, G.H.: A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509 (1957)
Hinton, A., Sennoune, R.S., Bond, S., Fang, M., Reuveni, M.G., Sahagian, G., Jay, D., Martinez-Zaguilan, R., Forgac, M.: Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J. Biol. Chem. 284, 16400–16408 (2009)
Sugarbaker, E.V.: The complex clinical model of metastasis. Med. Biol. 2, 235–278 (1981)
Tsujino, T., Yoshida, K., Nakayama, H., Ito, H., Shimosato, T., Tahara, R.: Alterations of oncogenes in metastatic tumors of human gastric carcinomas. Br. J. Cancer 62, 226–230 (1990)
Pasquet, V., Morisset, P., Ihammouine, S., Chepied, A., Aumailley, L., Berard, J.-B., Serive, B., Kaas, R., Lanneluc, I., Thiery, V., Lafferriere, M., Piot, J.-M., Patrice, T., Cadoret, J.-P., Picot, L.: Antiproliferative activity of violaxanthin isolated from bioguided fractionation of dunaliella tertiolecta extracts. Mar. Drugs 9, 819–831 (2011)
Motta, L.B., Furlan, C.M., Santos, D.Y.A.C., Salatino, M.L.F., Duarte-Almeida, J.M., Negri, G.E., De Carvalho, J.E., Ruiz, A.L.T.G., Cordeiro, I., Salatino, A.: Constituents and antiproliferative activity of extracts from leaves of Croton macrobothrys. Braz. J. Pharmacogn. 21, 972–977 (2011)
Taghizadeh, R.S.Z., Mahmoudi, M., Ahi, A., Emami, S.A.: Anti-proliferative effects of extracts from iranian artemisia species on cancer cell lines. Pharm. Biol. 49, 962–969 (2011)
Bardonemail, S., Foussard, V., Fournel, S., Loubat, A.: Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 181, 187–194 (2002)
Hong, J.-Y., Nam, J.-W., Seo, E.-K., Lee, S.K.: Daphnane diterpene esters with anti-proliferative activities against human lung cancer cells from Daphne genkwa. Chem. Pharm. Bull. 58, 234–237 (2010)
Paduch, R., Kandefer-Szerszeń, M., Trytek, M., Fiedurek, J.: Terpenes: substances useful in human healthcare. Arch. Immunol. Ther. Exp. J. 55, 315–327 (2007)
Acknowledgments
We wish to thank Chloé Chaillou for her technical help. This study was supported by the Bourse du Gouvernement Français (BGF) and the grants from the Western Indian Ocean Marine Science Association (WIOMSA) into the Marine Research Grant (MARG II).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fitahia, E.M., Raheriniaina, C.E., Bazin, M.A. et al. Anti-proliferative and Pro-apoptotic Effect of Dichloromethane Extract of Octopus vulgaris By-Products on Human Breast Cancer Cell Lines. Waste Biomass Valor 6, 237–242 (2015). https://doi.org/10.1007/s12649-014-9344-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-014-9344-1