Abstract
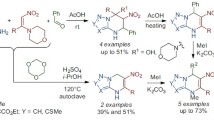
A preparative procedure for the synthesis of 1,4-dimethyl-3,5-dinitropyrazole by nitration of 1,4-dimethylpyrazole was developed. The reaction of 1,4-dimethyl-3,5-dinitropyrazole with dimethoxymethyl- (dimethyl)amine (N,N-dimethylformamide dimethyl acetal) gave (E)-N,N-dimethyl-2-(1-methyl-3,5-dinitropyrazol- 4-yl)ethenylamine. Acid hydrolysis of the latter afforded (1-methyl-3,5-dinitropyrazol-4-yl)acetaldehyde, and the reaction with sodium nitrite in hydrochloric acid led to formation of 2-hydroxymino-2-(1-methyl- 3,5-dinitropyrazol-4-yl)acetaldehyde. The corresponding O-methyloxime and phenylhydrazone reacted with K2CO3 to give 6-methyl-4-nitropyrazolo[4,3-d]isoxazole-3-carbaldehyde O-methyloxime and 1-methyl-3-nitro-4-(2-phenyl-2H-1,2,3-triazol-4-yl)pyrazol-5-ol, respectively. Treatment of (1-methyl-3,5-dinitropyrazol-4-yl)-acetaldehyde with benzenediazonium chloride gave (1-methyl-3,5-dinitropyrazol-4-yl)acetaldehyde phenylhydrazone which underwent intramolecular cyclization with replacement of the 5-nitro group by the action of K2CO3 in acetonitrile; in the reaction with K2CO3 in ethanol, the 5-nitro group was replaced by ethoxy.
Similar content being viewed by others
REFERENCES
Dalinger, I.L., Zaitsev, A.A., Shkineva, T.K., and Shevelev, S.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2004, p. 553.
Comprehensive Heterocyclic Chemistry. The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds, Katritzky, A.R., Ed., Oxford: Pergamon, 1984, vol. 5, p. 273.
Anderson, D.J. and Muchmore, C.R., J. Heterocycl. Chem., 1995, vol. 32, p. 1189; Holzer, W. and Hahn, K., J. Heterocycl. Chem., 2003, vol. 40, p. 303; Paul, S., Gupta, M., Gupta, R., and Loupyb, A., Tetrahedron Lett., 2001, vol. 42, p. 3827; Brown, K.J. and Meth-Cohn, O., Tetrahedron Lett., 1974, vol. 46, p. 4069; Koshelev, Yu.N., Reznichenko, A.V., Efros, L.S., and Kvitko, I.Ya., Zh. Org. Khim., 1973, vol. 9, p. 2201.
Shevelev, S.A. and Dalinger, I.L., Russ. J. Org. Chem., 1998, vol. 34, p. 1071.
Katritzky, A.R., Tarhan, H.O., and Terem, B., J. Chem. Soc., Perkin Trans. 2, 1975, p. 1632.
Yanovskaya, L.A., Yufit, S.S., and Kucherov, V.F., Izv. Akad. Nauk SSSR, Ser. Khim., 1960, p. 1246.
Bystrov, V.F., Grandberg, I.I., and Sharova, G.I., Zh. Obshch. Khim., 1965, vol. 35, p. 293.
Vinogradov, V.M., Dalinger, I.L., Starosotnikov, A.M., and Shevelev, S.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2001, p. 445; Vinogradov, V.M., Starosotnikov, A.M., and Shevelev, S.A., Mendeleev Commun., 2002, p. 198; Starosotnikov, A.M., Lobach, A.V., Kachala, V.V., Vinogradov, V.M., and Shevelev, S.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2003, p. 1690.
Vinogradov, V.M., Dalinger, I.L., Starosotnikov, A.M., and Shevelev, S.A., Mendeleev Commun., 2000, p. 140; Toste, F.D. and Steel, I.W.J., Org. Prep. Proced. Int., 1995, vol. 27, p. 576; Somei, M., Inoue, S., Tokutake, S., Yamada, F., and Kaneko, C., Chem. Pharm. Bull., 1981, vol. 29, p. 726; Arcari, M., Aveta, R., Brandt, A., Cecchetelli, L., Corsi, G.B., and Di Rella, M., Gazz. Chim. Ital., 1991, vol. 121, p. 499.
Borsche, W., Justus Liebigs Ann. Chem., 1912, vol. 390, p. 1; Kovendi, A. and Kircz, M., Chem. Ber., 1964, vol. 97, p. 1902; Reich, M.S. and Nicolaeva, V., Bull. Soc. Chim. Fr., 1919, p. 190.
Becher, J., Jorgensen, P.L., Pluta, K., Krake, N.J., and Falt-Hansen, B., J. Org. Chem., 1992, vol. 57, p. 2127.
Larina, L.I. and Lopyrev, V.A., Top. Heterocycl. Syst., 1996, p. 187.
Korlyukov, A.A., Starosotnikov, A.M., Lysenko, K.A., Shevelev, S.A., and Antipin, M.Yu., Izv. Ross. Akad. Nauk, Ser. Khim., 2003, p. 1985.
Starosotnikov, A.M., Vinogradov, V.M., Kachala, V.V., and Shevelev, S.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2002, p. 1399.
Hamper, B.C., Kurtzweil, M.L., and Beck, J.P., J. Org. Chem., 1992, vol. 57, p. 5680.
Marek, R. and Lycka, A., Curr. Org. Chem., 2002, p. 35.
Borsche, W., Chem. Ber., 1909, vol. 42, p. 601; Reich, M.S., Bull. Soc. Chim. Fr., 1917, p. 111; Prakash, A. and Gambhir, I.R., J. Indian. Chem. Soc., 1966, vol. 43, p. 529; Rozhkov, V.V., Vorob'ov, S.S., Lobatch, A.V., Kuvshinov, A.M., and Shevelev, S.A., Synth. Commun., 2002, vol. 32, p. 467.
Author information
Authors and Affiliations
Additional information
Dedicated to Full Member of the Russian Academy of Sciences N.S. Zefirov on His 70th Anniversary
__________
Translated from Zhurnal Organicheskoi Khimii, Vol. 41, No. 10, 2005, pp. 1538–1546.
Original Russian Text Copyright © 2005 by Zaitsev, Dalinger, Starosotnikov, Kachala, Strelenko, Shkineva, Shevelev.
Rights and permissions
About this article
Cite this article
Zaitsev, A.A., Dalinger, I.L., Starosotnikov, A.M. et al. Nitropyrazoles: XII. Transformations of the 4-Methyl Group in 1,4-Dimethyl-3,5-dinitropyrazole and Cyclization of the Transformation Products. Russ J Org Chem 41, 1507–1515 (2005). https://doi.org/10.1007/s11178-005-0374-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11178-005-0374-9