Abstract
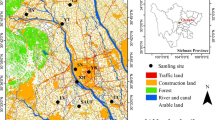
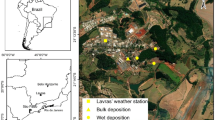
Nutrients that fall on the ground from the atmosphere represent a minor component of the total nitrogen (N) input to soils, especially when compared with agricultural, civil and industrial inputs (i.e. sewage treatment plants or sewage systems, fertilizer and manure applications). However, integrating all nitrogen forms, processes and scales can represent a breakthrough challenge for the understanding and the management of the N cycle. A monitoring experiment was set up to collect wet atmospheric depositions in a human-impacted area with multiple land uses, representing different emission sources. Rainwater collection was executed in the surroundings of Milan, in northern Italy, starting from February 2017 to February 2019. The presence of N compounds and their temporal variations in rainwater are consistent with pollution coming from local anthropogenic emission sources of nitrogen oxides and ammonia, mainly related to the use of the heating systems in the cold seasons and the spreading of fertilizers and manure on agricultural fields. Consequently, the total amount of N wet depositions ranges between 14 and about 30 kg/ha yr in the study area. As leaching of N compounds from soils generally increases at deposition rates higher than about 10 kg(N)/ha yr, this work suggests that the N atmospheric input to soils could not be neglected when evaluating the impacts of N sources to terrestrial and aquatic ecosystems, as well as to groundwater resources. This highlights the need of wisely integrating air, soil and water policies for minimising the risk to deteriorate surficial ecosystems and groundwater.










Similar content being viewed by others
References
Amato, F., Alastuey, A., Karanasiou, A., Lucarelli, F., Nava, S., Calzolai, G., et al. (2016). AIRUSE-LIFE+: A harmonized PM speciation and source apportionment in five southern European cities. Atmospheric Chemistry and Physics, 16(5), 3289–3309. https://doi.org/10.5194/acp-16-3289-2016.
Balestrini, R., Galli, L., & Tartari, G. (2000). Wet and dry atmospheric deposition at prealpine and alpine sites in northern Italy. Atmospheric Environment, 34(9), 1455–1470. https://doi.org/10.1016/S1352-2310(99)00404-5.
Berkowicz, R., Palmgren, F., Hertel, O., & Vignati, E. (1996). Using measurements of air pollution in streets for evaluation of urban air quality - Meterological analysis and model calculations. Science of the Total Environment, 189-190, 259–265. https://doi.org/10.1016/0048-9697(96)05217-5.
Bland, J. M., & Altman, D. G. (1999). Measuring agreement in method comparison studies. Statistical Methods in Medical Research, 8, 135–160.
Bobbink R., Hettelingh J.P. (2011). Review and revisions of empirical critical loads and dose–response relationships. Proceedings of an expert workshop, Noordwijkerhout 23–24 June 2010, Bilthoven, the Netherlands, RIVM.
Brimblecombe, P. (2003). 8.14 - the global sulfur cycle. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry (pp. 645–682). Oxford: Pergamon. https://doi.org/10.1016/B0-08-043751-6/08134-2.
Brunetti, M., Buffoni, L., Maugeri, M., & Nanni, T. (2000). Precipitation intensity trends in northern Italy. International Journal of Climatology: A Journal of the Royal Meteorological Society, 20(9), 1017–1031.
Buoli, M., Grassi, S., Caldiroli, A., Carnevali, G. S., Mucci, F., Iodice, S., Cantone, L., Pergoli, L., & Bollati, V. (2018). Is there a link between air pollution and mental disorders? Environment International, 118, 154–168. https://doi.org/10.1016/j.envint.2018.05.044.
Carnevale, C., Decanini, E., & Volta, M. (2008). Design and validation of a multiphase 3-D model to simulate tropospheric pollution. Science of the Total Environment, 390, 166–176. https://doi.org/10.1016/j.scitotenv.2007.09.017.
Celle-Jeanton, H., Travi, Y., Loÿe-Pilot, M.-D., Huneau, F., & Bertrand, G. (2009). Rainwater chemistry at a Mediterranean inland station (Avignon, France): Local contribution versus long-range supply. Atmospheric Research, 91, 118–126. https://doi.org/10.1016/j.atmosres.2008.06.003.
Ceriani, M., & Carelli, M. (2003). Carta delle precipitazioni medie, massime e minime annue del territorio alpino della Regione Lombardia (registrate nel periodo 1891–1990). Pubblicazione Regione Lombardia.
Charlson, R. J., & Rhode, J. (1982). Factors controlling the acidity of natural rainwater. Nature, 295, 683–685.
Deusdará, K. R. L., Forti, M. C., Borma, L. S., Menezes, R. S. C., Lima, J. R. S., & Ometto, J. P. H. B. (2017). Rainwater chemistry and bulk atmospheric deposition in a tropical semiarid ecosystem: The Brazilian Caatinga. Journal of Atmospheric Chemistry, 74(1), 71–85. https://doi.org/10.1007/s10874-016-9341-9.
Dosio, A., Galmarini, S., & Graziani, G. (2002). Simulation of the circulation and related photochemical ozone dispersion in the Po plains (northern Italy): Comparison with the observations of a measuring campaign. Journal of Geophysical Research, 107(D18), 8189. https://doi.org/10.1029/2000JD000046.
EEA – European Environment Agency (2018). European Union emission inventory report 1990–2016 under the UNECE Convention on Long-range Transboundary Air Pollution (LRTAP). EEA Report No 6/2018, pp. 150, doi: https://doi.org/10.2800/571876
EMEP/CEIP (2018). EMEP emissions data. European monitoring and evaluation Programme/Centre on emission inventories and projections. Available at: http://www.ceip.at/
ESA (2019a). Nitrogen dioxide pollution mapped. European Space Agency, news released on 12 March 2019. Available at: http://www.esa.int/Our_Activities/Observing_the_Earth/Copernicus/Sentinel-5P/Nitrogen_dioxide_pollution_mapped
ESA (2019b). The air we breathe. European Space Agency, news released on 16 May 2019. Available at: http://www.esa.int/Our_Activities/Observing_the_Earth/Copernicus/Sentinel-5P/The_air_we_breathe2
European Commission (1991). Council Directive 91/676/EEC concerning the protection of waters against pollution caused by nitrates from agricultural sources, (Nitrate Directive). OJ L 375, 31 December 1991, pp. 1–8.
European Commission (2008). Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. OJ L 152, 11 June 2008, pp. 1–44.
Galloway, J. N., Lickens, G. E., Keene, W. C., & Miller, J. M. (1982). The composition of precipitation in remote areas of the world. Journal of Geophysical Research, 87, 8771–8786.
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., Martinelli, L. A., Seitzinger, S. P., & Sutton, M. A. (2008). Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science, 320(5878), 889–892. https://doi.org/10.1126/science.1136674.
Gómez-Carracedo, M. P., Andrade, J. M., Ballabio, D., Prada-Rodríguez, D., Muniategui-Lorenzo, S., Consonni, V., Piñeiro-Iglesias, M., & López-Mahía, P. (2015). Impact of medium-distance pollution sources in a Galician suburban site (NW Iberian peninsula). Science of the Total Environment, 512-513, 114–124. https://doi.org/10.1016/j.scitotenv.2015.01.029.
Grimshaw, H. J., & Dolske, D. A. (2002). Rainfall concentrations and wet atmospheric deposition of phosphorus and other constituents in Florida, U.S.A. Water, Air, and Soil Pollution, 137(1–4), 117–140. https://doi.org/10.1023/A:1015505624739.
Ham, Y. S., & Tamiya, S. (2006). Contribution of dissolved organic nitrogen deposition to total dissolved nitrogen deposition under intensive agricultural activities. Water Air Soil Pollution, 178(1–4), 5–13. https://doi.org/10.1007/s11270-006-9109-y.
ISPRA - Istituto Superiore per la Protezione e la Ricerca Ambientale (2016). Focus su inquinamento atmosferico nelle aree urbane ed effetti sulla salute, Stato dell’ambiente 68/2016.
Keene, W. C., Pszenny, A. P., Galloway, J. N., & Hawley, M. E. (1986). Sea salt corrections and interpretations of constituent ratios in marine precipitation. Journal of Geophysical Research, 91, 6647–6658.
Kennedy, F. (2001). How extensive are the impacts of nitrogen pollution in Great Britain’s forests? Forest Research Annual Report, 2001/2, 66–75.
Kim, K. H., Yun, S. T., Kim, H., & Kim, J. (2015). Determination of natural backgrounds and thresholds of nitrate in South Korean groundwater using model-based statistical approaches. Journal of Geochemical Exploration, 148, 196–205. https://doi.org/10.1016/j.gexplo.2014.10.001.
Lee, D. S., Kingdon, R. D., Pacyna, J. M., Bouwman, A. F., & Tegen, I. (1999). Modelling base cations in Europe—Sources, transport and deposition of calcium. Atmospheric Environment, 33(14), 2241–2256. https://doi.org/10.1016/S1352-2310(98)00169-1.
Magari, M. T. (2002). Statistics for laboratory method comparison studies. BioPharm International, 15(1), 28–32.
Meyer, S. T., Koch, C., & Weisseret, W. W. (2015). Towards a standardized rapid ecosystem function assessment (REFA). Trends in Ecology & Evolution, 30, 390–397. https://doi.org/10.1016/j.tree.2015.04.006.
Moreda-Piñeiro, J., Alonso-Rodríguez, E., Moscoso-Pérez, C., Blanco-Heras, G., Turnes-Carou, I., López-Mahía, P., Muniategui-Lorenzo, S., & Prada-Rodríguez, D. (2014). Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmospheric Environment, 88, 30–38. https://doi.org/10.1016/j.atmosenv.2014.01.067.
Mosello, R., & Tartari, G. A. (1992). Formiate and acetate in wet deposition at Pallanza (NW Italy) in relation to major ion concentrations. Water, Air and Soil Pollution, 63, 397–409.
NASA Hyperwall (2018). Nitrogen dioxide from Aura/OMI, 2017-2018. Available at: https://svs.gsfc.nasa.gov/30986
Naselli-Flores, L. (2010). Mediterranean climate and eutrophication of reservoirs: Limnological skills to improve management. In A. Ansari, G. S. Singh, G. Lanza, & W. Rast (Eds.), Eutrophication: Causes, consequences and control (pp. 131–142). Dordrecht: Springer.
Ochoa-Hueso, R., Allen, E. B., Branquinho, C., Cruz, C., Dias, T., Fenn, M. E., et al. (2011). Nitrogen deposition effects on Mediterranean-type ecosystems: An ecological assessment. Environmental Pollution, vol., 159(10), 2265–2279. https://doi.org/10.1016/j.envpol.2010.12.019.
Ordóñez, C., Richter, A., Steinbacher, M., Zellweger, C., Nüß, H., Burrows, J. P., & Prévôt, A. S. H. (2006). Comparison of 7 years of satellite-borne and ground-based tropospheric NO2 measurements around Milan, Italy. Journal of Geophysical Research, 111, D05310. https://doi.org/10.1029/2005JD006305.
Panettiere, P., Cortecci, G., Dinelli, E., Bencini, A., & Guidi, M. (2000). Chemistry and sulfur isotopic composition of precipitation at Bologna, Italy. Applied Geochemistry, 15(10), 1455–1467. https://doi.org/10.1016/S0883-2927(00)00012-3.
Panno, S. V., Kelly, W. R., Martinsek, A. T., & Hackley, K. C. (2006). Estimating background and threshold nitrate concentrations using probability graphs. Ground Water, 44(5), 697–709. https://doi.org/10.1111/j.1745-6584.2006.00240.x.
Payne, R. J., Dise, N. B., Stevens, C. J., Gowing, D. J., & Partners, B. E. G. I. N. (2013). Impact of nitrogen deposition at the species level. PNAS, 110(3), 984–987. https://doi.org/10.1073/pnas.1214299109.
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644.
Rattigan, O. V., Civerolo, K. L., & Felton, H. D. (2017). Trends in wet precipitation, particulate, and gas-phase species in New York State. Atmospheric Pollution Research, 8(6), 1090–1102. https://doi.org/10.1016/j.apr.2017.04.007.
Rogora, M., Arisci, S., & Marchetto, A. (2012). The role of nitrogen deposition in the recent nitrate decline in lakes and rivers in Northern Italy. Science of the Total Environment, 417–418, 214–223. https://doi.org/10.1016/j.scitotenv.2011.12.067.
Rogora, M., Colombo, L., Marchetto, A., Mosello, R., & Steingruber, S. (2016). Temporal and spatial patterns in the chemistry of wet deposition in Southern Alps. Atmospheric Environment, 146, 44–54. https://doi.org/10.1016/j.atmosenv.2016.06.025.
Schindler, D. W. (2006). Recent advances in the understanding and management of eutrophication. Limnology and Oceanography, 51(1), 356–363. https://doi.org/10.4319/lo.2006.51.1_part_2.0356.
Sorichetta, A., Ballabio, C., Masetti, M., Robinson Jr., G. R., & Sterlacchini, S. (2013). A comparison of data-driven groundwater vulnerability assessment methods. Ground Water, 51(6), 866–879. https://doi.org/10.1111/gwat.12012.
Steingruber, S. (2015). Acidifying deposition in southern Switzerland. Monitoring, maps and trends 1988–2013. Ufficio dell’aria, del clima e delle energie rinnovabili (p. 60). Bellinzona: Dipartimento del territorio del Canton Ticino.
Stevenazzi, S., Nghiem, S. V., & Masetti, M. (2015). Urban impacts on air quality observed with remote sensing and ground station data from the Po Plain Field Campaign. In IEEE Geoscience and Remote Sensing Symposium (pp. 73–75). Milan: ISBN: 978-1-4799-7928-8.
Stevenazzi, S., Masetti, M., & Beretta, G. P. (2017). Groundwater vulnerability assessment: From overlay methods to statistical methods in the Lombardy plain area. Acque Sotterranee - Italian Journal of Groundwater, 6, 17–27. https://doi.org/10.7343/as-2017-276.
Sutton, M. A., Howard, C. M., Erisman, J. W., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., & Grizzeti, B. (2011). The European nitrogen assessment: Sources, effects and policy perspectives (p. 612). Cambridge University Press.
Van Damme, M., Clarisse, L., Whitburn, S., Hadji-Lazaro, J., Hurtmans, D., Clerbaux, C., & Coheur, P.-F. (2018). Industrial and agricultural ammonia point sources exposed. Nature, 564(7734), 99. https://doi.org/10.1038/s41586-018-0747-1.
Vet, R., Artz, R. S., Carou, S., Shaw, M., Ro, C.-U., Aas, W., Baker, A., Bowersox Van, C., Dentener, F., Galy-Lacaux, C., Hou, A., Pienaar, J. J., Gillett, R., Forti, M. C., Gromov, S., Hara, H., Khodzher, T., Mahowald, N. M., Nickovic, S., Rao, P. S. P., & Reid, N. W. (2014). A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmospheric Environment, 93, 3–100. https://doi.org/10.1016/j.atmosenv.2013.10.060.
Williams, J. J., Lynch, J. A., Saros, J. E., & Labou, S. G. (2017). Critical loads of atmospheric N deposition for phytoplankton nutrient limitation shifts in western US mountain lakes. Ecosphere, 8(10), e01955. https://doi.org/10.1002/ecs2.1955.
WMO/GAW (2004). Manual for the GAW Precipitation Chemistry Programme: Guidelines, data quality objectives and standard operating procedures. No. 160. World Meteorological Organization/Global Atmosphere Watch, Geneva, Switzerland.
Xiao, H.-W., Xiao, H.-Y., Long, A.-M., Wang, Y.-L., & Liu, C.-Q. (2013). Chemical composition and source apportionment of rainwater at Guiyang SW China. Journal of Atmospheric Chemistry, 70, 269–281. https://doi.org/10.1007/s10874-013-9268-3.
Yang, F., Tan, J., Shi, Z. B., Cai, Y., He, K., Ma, Y., Duan, F., Okuda, T., Tanaka, S., & Chen, G. (2012). Five-year record of atmospheric precipitation chemistry in urban Beijing, China. Atmospheric Chemistry and Physics, 12, 2025–2035. https://doi.org/10.5194/acp-12-2025-2012.
Acknowledgements
The authors wish to acknowledge the colleagues of the Department of Earth Sciences “A. Desio” of the University of Milan, for their help in collecting the rainwater samples.
Funding
This work received support from the Italian Ministry of Education (MIUR) through the project ‘Dipartimenti di Eccellenza 2017’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
1.1 Appendix 1
Microwave plasma atomic emission spectroscopy (MP-AES) and ion chromatography (ICS) methods in measuring major cation concentrations have been compared applying the Bland-Altman graphical technique (Bland and Altman 1999), which allows assessing the relative agreement between two laboratory analytical methods that measure the same chemical substance (Magari 2002).
Figure A1a shows the measurements obtained by ICS plotted against those obtained through MP-AES, to assess visually how well the methods agree: data should lie along a straight line passing through the origin in a 45° angle (i.e. equality line). But, it seems that ICS results are slightly higher than MP-AES results. However, this plot can be misleading: the greater the range of measurements, the better the agreement will appear to be. Instead, measuring the differences between the two methods for each measurement plotted against their means and adding the limits of agreement (Fig. A1b) is a better way of assessing the relationship, as it clearly shows the pattern of the individual differences. The mean difference is 0.07 mg/L, and the standard deviation of the differences is 0.48 mg/L. Thus, the limits of agreement with a 95% confidence level are − 0.86 mg/L and 1.01 mg/L (dashed lines in Fig. A1b). Nonetheless, Fig. A1b shows an increase in variability of the differences as the magnitude of the measurement increases. Thus, a logarithmic (i.e. natural logarithmic) transformation of both measurements has been applied.
Figure A2 shows the logarithmic transformed data and the difference versus mean plot with superimposed 95% limits of agreement. The range of values has been reduced and data lie along the ‘equality line’ (Fig. A2a). The mean difference (log ICS – log MP-AES) is 0.03 with 95% limits of agreement − 0.071 and 0.076 (Fig. A2b). Thus, it is possible to assure the agreement between ICS and MP-AES methods for measuring major cation concentration and that the two measurement methods can be used interchangeably.
It is important to note that instrumental errors are considered within the range of variability of differences between the two methods.
1.2 Appendix 2
(a) Ionic Balance
The completeness of measured parameters and the principle of electroneutrality in precipitation were checked through the ratio of total anions to that of cations. This ratio is expressed as (WMO/GAW 2004; Eq. A1):
with CE and AE being the sum of cation and anion equivalents (meq/L), respectively. CE is the sum of Ca2+, Na+, Mg2+, K+, NH4+ and H+. AE is the sum of NO3−, NO2−, SO42−, Cl− and F−. CE and AE are calculated as (WMO/GAW 2004; Eqs. A2 and A3):
where CCi and CAi are the concentrations of the ith cation (C) or anion (A) in mg/L, Eq.Wt. is the equivalent weight of the ith cation (C) or anion (A), 10(6-pH) is the H+ concentration in meq/L.
The acceptability criterion was set as BAL ≤ ± 20% to comply with the requirements of the Global Atmosphere Watch Program for precipitation chemistry (WMO/GAW 2004) and according to previous works on precipitation quality (e.g. Balestrini et al. 2000; Yang et al. 2012).
(b) Electric Conductivity Balance
Measured and calculated electric conductivities (EC) are compared according to Eq. A4 (WMO/GAW 2004).
where ECC is the calculated electric conductivity and EC is the measured electric conductivity, both expressed as μS/cm. For dilute solutions, the total (calculated) conductivity can be calculated in μS/cm from the molar concentrations and molar ionic conductances of the individual ions, as follows (WMO/GAW 2004; Eq. A5):
where ECC is the calculated conductivity (μS/cm), ci is the ionic concentration of the ith ion (mmol/L) and Λi° the molaric ionic conductance of the ith ion (Scm2/mol) at infinite solution and 25 °C. Thus (Eq. A6):
where 10(3-pH) expresses the concentration of H+.
The acceptability criterion was set as ΔEC ≤ ±30% to comply with the requirements of the Global Atmosphere Watch Program for precipitation chemistry (WMO/GAW 2004) and according to previous works on precipitation quality (e.g. Balestrini et al. 2000; Yang et al. 2012).
(c) Marine Inputs
The sea salt fraction (SSF) and the non-sea salt fraction (NSSF) are calculated according to Keene et al. (1986); WMO/GAW 2004) by comparing ionic ratios in rainwater and seawater using sodium as the reference species (e.g. Moreda-Piñeiro et al. 2014; Deusdará et al. 2017), following Eqs. A7 and A8.
where X is the concentration of ions as measured in rainwater, Xsea is the concentration of the sea water ions and Nasea is the concentration of Na+ taken as a reference in sea water. All concentrations are expressed in meq/L.
(d) Neutralisation Factors
The acidity of rainwater potentially originates from the dissociation of nitric and sulphuric acid anions (NO3− and SO42−) that are neutralised by alkaline species, such as Ca2+, Mg2+, K+ and NH4+ as well as on the neutralisation reactions among them (e.g. Moreda-Piñeiro et al. 2014; Deusdará et al. 2017). The neutralisation factor (NF) is expressed as shown in Eq. A9.
where [X] is the concentration of the species responsible for neutralisation (Ca2+, Mg2+, K+, NH4+). The volume weighted mean for each species, expressed as meq/L, is used for the calculation of the neutralisation factors.
(e) Descriptive Statistics
Descriptive statistics are calculated for ionic concentrations, EC and pH values: arithmetic mean, volume weighted mean, median, minimum and maximum values and standard deviation. Volume weighted mean (VWM) was calculated by using Eq. A10. It allows taking into account the effect of dilution by the rain amount and is useful in comparative studies.
where [Xi] is the concentration of ion X or the value of parameter X (i.e. pH and EC), vi is the water sample volume and N is the number of samples. Concentration can be expressed as mg/L, meq/L or μeq/L. pH has no unit of measure. EC is expressed as μS/cm.
Rights and permissions
About this article
Cite this article
Stevenazzi, S., Camera, C.A.S., Masetti, M. et al. Atmospheric Nitrogen Depositions in a Highly Human-Impacted Area. Water Air Soil Pollut 231, 276 (2020). https://doi.org/10.1007/s11270-020-04613-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04613-y