ABSTRACT
Purpose
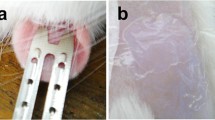
To evaluate the feasibility of coating formulated recombinant human erythropoietin alfa (EPO) on a titanium microneedle transdermal delivery system, ZP-EPO, and assess preclinical patch delivery performance.
Methods
Formulation rheology and surface activity were assessed by viscometry and contact angle measurement. EPO liquid formulation was coated onto titanium microneedles by dip-coating and drying. Stability of coated EPO was assessed by SEC-HPLC, CZE and potency assay. Preclinical in vivo delivery and pharmacokinetic studies were conducted in rats with EPO-coated microneedle patches and compared to subcutaneous EPO injection.
Results
Studies demonstrated successful EPO formulation development and coating on microneedle arrays. ZP-EPO patch was stable at 25°C for at least 3 months with no significant change in % aggregates, isoforms, or potency. Preclinical studies in rats showed the ZP-EPO microneedle patches, coated with 750 IU to 22,000 IU, delivered with high efficiency (75–90%) with a linear dose response. PK profile was similar to subcutaneous injection of commercial EPO.
Conclusions
Results suggest transdermal microneedle patch delivery of EPO is feasible and may offer an efficient, dose-adjustable, patient-friendly alternative to current intravenous or subcutaneous routes of administration.






Similar content being viewed by others
REFERENCES
Centers for Disease Control and Prevention (CDC). National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: U.S. Department of Health and Human Services (HHS), CDC; 2010.
EPOGEN® (epoetin alfa) United States Prescribing Information (USPI).
Park K, Kwon IC, Park K. Oral protein delivery: current status and future prospect. React Funct Polym. 2011;71:280–7.
Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS Pharm Sci Tech. 2011;12:431–41.
Bachhav YG, Summer S, Heinrich A, Bragagna T, Boehler C, Kalia YN. Minimally invasive delivery of peptides and proteins across the skin using P.L.E.A.S.E.® technology. AAPS J. 2008;10(S2):1168.
Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, Zivin I, Phillip M. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22:550–5.
Badkar A, Smith A, Eppstein J, Banga A. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24:1389–95.
Badran MM, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci. 2009;36:511–23.
Burton SA, Ng CY, Simmers R, Moeckly C, Brandwein D, Gilbert T, Johnson N, Brown K, Alston T, Prochnow G, Siebenaler K, Hansen K. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm Res. 2011;28:31–40.
Gupta J, Felner EI, Prausnitz MR. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13:451–6.
Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques. J Pharm Sci. 2010;99:1931–41.
Fukushima K, Ise A, Morita H, Hasegawa R, Ito Y, Sugioka N, Takada K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm Res. 2011;28:7–21.
Migalska K, Morrow DI, Garland MJ, Thakur R, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28:1919–30.
Lee JW, Choi SO, Felner EI, Prausnitz MR. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;18:531–9.
Gill HS, Söderholm J, Prausnitz MR, Sällberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–4.
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–64.
Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang D, Daddona PE. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–11.
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70.
Cosman F, Lane NE, Bolognese M, Zanchetta J, Garcia-Hernandez PA, Sees K, Matriano JA, Gaumer K, Daddona PE. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrin Metab. 2010;95:151–8.
Daddona PE, Matriano JA, Mandema J, Maa Y-F. Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28:159–65.
Sathyan G, Sun Y-N, Weyers R, Daddona P, Staehr P, Gupta S. Macroflux® desmopressin transdermal delivery system: pharmacokinetic and pharmacodynamic evaluation in healthy volunteers. AAPS J. 2004;6(S1):665.
Ito Y, Yoshimitsu J, Shiroyama K, Sugioka N, Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14:255–61.
Ito Y, Shiroyamaa K, Yoshimitsua J, Ohashia Y, Sugiokaa N, Takadaa K. Pharmacokinetic and pharmacodynamic studies following percutaneous absorption of erythropoietin micropiles to rats. J Control Release. 2007;121:176–80.
Ito Y, Hasegawa R, Fukushima K, Sugioka N, Takada K. Self-dissolving micropile array chip as percutaneous delivery system of protein drug. Biol Pharm Bull. 2010;33:683–90.
European Pharmacopeia 7th edition. European Department for the quality of the medicines. Strasbourg. France (2010).
Cormier M, Neukermans AP, Block B, Theeuwes FT, Amkraut A. A device for enhancing transdermal agent delivery or sampling. EP0914178B1, (2003).
Ameri M, Fan SC, Maa YF. Parathyroid hormone PTH(1-34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm Res. 2010;27:303–13.
Principles of Laboratory Animal Care. NIH publication #85-23, revised in (1985).
Guide for the Care and Use of Laboratory Animals (National Research Council). National Academy Press. Washington DC (1996).
Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharm Exp Thera. 1944;82:377–90.
Ameri M, Daddona PE, Maa Y-F. Demonstrated solid-state stability of parathyroid hormone PTH (1-34) coated on a novel transdermal Microneedle delivery system. Pharm Res. 2009;26:2454–63.
Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and Pharmacodynamic modelling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharm Exp Thera. 2006;319:1297–306.
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35:1672–8.
Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28:107–16.
ACKNOWLEDGMENTS & DISCLOSURES
The authors would like to thank Joseph A. Bravo, James A. Matriano for assistance in the pharmacokinetic studies, Perry Weissburg, Shelly Fan, Scott Sellers for their contribution to the analytical analyses and Kenneth Chan for formulation preparation and SEM analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, E.E., Ameri, M., Wang, X. et al. Erythropoietin-Coated ZP-Microneedle Transdermal System: Preclinical Formulation, Stability, and Delivery. Pharm Res 29, 1618–1626 (2012). https://doi.org/10.1007/s11095-012-0674-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0674-z