Abstract
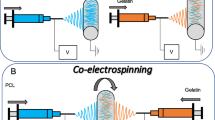
Four tubular membranes based on PLA and gelatin with different arrangements (PLA, gelatin, PLA-gelatin Blend and PLA/gelatin Core/Shell) were prepared by the electrospinning technique, the gelatin within the materials was crosslinked with genipin and all the meshes were characterized by SEM, ATR-FTIR, TGA, DSC, XRD and mechanical tests, also a viability essay and a confocal microscopy of HUVECs in contact with the materials were carried out. A 50% decrement of cellular viability was observed in the fibers with Core/Shell structure since the first day of culture compared with the tissue culture polystyrene, due to solubilization of non-crosslinked gelatin.











Similar content being viewed by others
References
Cameron A, Davis KB, Green G, Schaff HV (1996) Coronary bypass surgery with internal-thoracic-artery grafts—effects on survival over a 15-year period. N Engl J Med 334(4):216–220. https://doi.org/10.1056/NEJM199601253340402
Gaudino M, Benedetto U, Fremes S et al (2018) Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 378(22):2069–2077. https://doi.org/10.1056/nejmoa1716026
Serruys PW, Unger F, Sousa JE et al (2001) Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 344(15):1117–1124. https://doi.org/10.1056/NEJM200104123441502
Awad NK, Niu H, Ali U, Morsi YS, Lin T (2018) Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes (Basel). 8(1):1–26. https://doi.org/10.3390/membranes8010015
Kharazi AZ, Atari M, Vatankhah E, Javanmard SH (2018) A nanofibrous bilayered scaffold for tissue engineering of small-diameter blood vessels. Polym Adv Technol 29(12):3151–3158. https://doi.org/10.1002/pat.4437
Raines EW (2000) The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 81(3):173–182
Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP et al (2008) Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29(19):2907–2914. https://doi.org/10.1016/j.biomaterials.2008.03.034
Kai D, Liow SS, Loh XJ (2015) Biodegradable polymers for electrospinning: towards biomedical applications. Mater Sci Eng, C 45:659–670. https://doi.org/10.1016/j.msec.2014.04.051
Strobel HA, Calamari EL, Beliveau A, Jain A, Rolle MW (2018) Fabrication and characterization of electrospun polycaprolactone and gelatin composite cuffs for tissue engineered blood vessels. J Biomed Mater Res—Part B Appl Biomater 106(2):817–826. https://doi.org/10.1002/jbm.b.33871
Tsuji H (2014) Poly (lactic acid). https://doi.org/10.1002/9780470649848
Park H, Radisic M, Lim JO, Chang BH, Vunjak-Novakovic G (2005) A novel composite scaffold for cardiac tissue engineering. Vitr Cell Dev Biol Anim. 41(7):188. https://doi.org/10.1290/0411071.1
Mi HY, Jing X, Li ZT, Lin YJ, Thomson JA, Turng LS (2019) Fabrication and modification of wavy multicomponent vascular grafts with biomimetic mechanical properties, antithrombogenicity, and enhanced endothelial cell affinity. J Biomed Mater Res Part B Appl Biomater 1–12. https://doi.org/10.1002/jbm.b.34333
Swarnalatha B, Nair SL, Shalumon KT et al (2013) Poly (lactic acid)-chitosan-collagen composite nanofibers as substrates for blood outgrowth endothelial cells. Int J Biol Macromol 58:220–224. https://doi.org/10.1016/j.ijbiomac.2013.03.060
Lynn AK, Yannas IV, Bonfield W (2004) Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B Appl Biomater 71(2):343–354. https://doi.org/10.1002/jbm.b.30096
Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG (1998) Repair of craniotomy defects using bone marrow stromal cells. Transplantation 66(10):1272–1278. https://doi.org/10.1097/00007890-199811270-00002
Peter M, Binulal NS, Nair SV, Selvamurugan N, Tamura H, Jayakumar R (2010) Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem Eng J 158(2):353–361. https://doi.org/10.1016/j.cej.2010.02.003
Dreesmann L, Ahlers M, Schlosshauer B (2007) The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials 28(36):5536–5543. https://doi.org/10.1016/j.biomaterials.2007.08.040
Hong Y, Gong Y, Gao C, Shen J (2008) Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J Biomed Mater Res Part A. 85(3):628–637. https://doi.org/10.1002/jbm.a.31603
Aldana AA, Abraham GA (2017) Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm 523(2):441–453. https://doi.org/10.1016/j.ijpharm.2016.09.044
Ullm S, Krüger A, Tondera C et al (2014) Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties. Biomaterials 35(37):9755–9766. https://doi.org/10.1016/j.biomaterials.2014.08.023
Piris MA, Mollejo M, Campo E, Menárguez J, Flores T, Isaacson PG (1998) A marginal zone pattern may be found in different varieties of non-Hodgkin’s lymphoma: the morphology and immunohistology of splenic involvement by B-cell lymphomas simulating splenic marginal zone lymphoma. Histopathology 33(3):230–239. https://doi.org/10.1046/j.1365-2559.1998.00478.x
Manickam B, Sreedharan R, Elumalai M (2014) ‘Genipin’—the natural water soluble cross-linking agent and its importance in the modified drug delivery systems: an overview. Curr Drug Deliv 11(1):139–145. https://doi.org/10.2174/15672018113106660059
Liang HC, Chang WH, Liang HF, Lee MH, Sung HW (2004) Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J Appl Polym Sci 91(6):4017–4026. https://doi.org/10.1002/app.13563
Butler MF, Ng YF, Pudney PDA (2003) Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci, Part A: Polym Chem 41(24):3941–3953. https://doi.org/10.1002/pola.10960
Wang DK, Varanasi S, Fredericks PM et al (2013) FT-IR characterization and hydrolysis of PLA-PEG-PLA based copolyester hydrogels with short PLA segments and a cytocompatibility study. J Polym Sci, Part A: Polym Chem 51(24):5163–5176. https://doi.org/10.1002/pola.26930
2001—Thomson Learning, Inc.—Pavia, Lampman, Kriz - Introduction to Spectroscopy third edition.pdf
Hashim DM, Man YBC, Norakasha R, Shuhaimi M, Salmah Y, Syahariza ZA (2010) Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem 118(3):856–860. https://doi.org/10.1016/j.foodchem.2009.05.049
Daniel-Da-Silva AL, Salgueiro AM, Trindade T (2013) Effects of Au nanoparticles on thermoresponsive genipin-crosslinked gelatin hydrogels. Gold Bull. 46(1):25–33. https://doi.org/10.1007/s13404-012-0078-1
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta Bioenerget 1767(9):1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Di Tommaso S, David H, Gomar J, Leroy F, Adamo C (2014) From iridoids to dyes: a theoretical study on genipin reactivity. RSC Adv 4(22):11029–11038. https://doi.org/10.1039/c3ra47159d
Touyama R, Takeda Y, Inoue K et al (2011) Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem Pharm Bull 42(3):668–673. https://doi.org/10.1248/cpb.42.668
Devi N, Maji TK (2010) Genipin crosslinked microcapsules of gelatin a and κ-carrageenan polyelectrolyte complex for encapsulation of neem (azadirachta indica a.juss.) seed oil. Polym Bull 65(4):347–362. https://doi.org/10.1007/s00289-010-0246-5
Moshiul Alam AKM, Beg MDH, Mina MF, Mamun AA, Bledzki AK (2015) Degradation and stability of green composites fabricated from oil palm empty fruit bunch fiber and polylactic acid: effect of fiber length. J Compos Mater 49(25):3103–3114. https://doi.org/10.1177/0021998314560219
Frone AN, Berlioz S, Chailan JF, Panaitescu DM (2013) Morphology and thermal properties of PLA-cellulose nanofibers composites. Carbohydr Polym 91(1):377–384. https://doi.org/10.1016/j.carbpol.2012.08.054
Jalaja K, Naskar D, Kundu SC, James NR (2015) Fabrication of cationized gelatin nanofibers by electrospinning for tissue regeneration. RSC Adv 5(109):89521–89530. https://doi.org/10.1039/c5ra10384c
Feldstein MM, Roos A, Chevallier C, Creton C, Dormidontova EE (2003) Relation of glass transition temperature to the hydrogen bonding degree and energy in poly(N-vinyl pyrrolidone) blends with hydroxyl-containing plasticizers: 3. Analysis of two glass transition temperatures featured for PVP solutions in liquid poly(ethyle). Polymer Guildf 44(6):1819–1834. https://doi.org/10.1016/s0032-3861(03)00046-6
Bouakaz BS, Pillin I, Habi A, Grohens Y (2018) Synergy between fillers in organomontmorillonite/graphene-PLA nanocomposites. Appl Clay Sci 2015(116–117):69–77. https://doi.org/10.1016/j.clay.2015.08.017
Mathew AP, Oksman K, Sain M (2006) The effect of morphology and chemical characteristics of cellulose reinforcements on the crystallinity of polylactic acid. J Appl Polym Sci 101(1):300–310. https://doi.org/10.1002/app.23346
Inai R, Kotaki M, Ramakrishna S (2005) Structure and properties of electrospun PLLA single nanofibres. Nanotechnology 16(2):208–213. https://doi.org/10.1088/0957-4484/16/2/005
Ercolani E, Del Gaudio C, Bianco A (2013) Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J Tissue Eng Regen Med 2012:861–888. https://doi.org/10.1002/term
Wong SC, Baji A, Leng S (2008) Effect of fiber diameter on tensile properties of electrospun poly(ε-caprolactone). Polymer (Guildf). 49(21):4713–4722. https://doi.org/10.1016/j.polymer.2008.08.022
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV (2005) In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 26(36):7616–7627. https://doi.org/10.1016/j.biomaterials.2005.05.036
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leyva-Verduzco, A.A., Castillo-Ortega, M.M., Chan-Chan, L.H. et al. Electrospun tubes based on PLA, gelatin and genipin in different arrangements for blood vessel tissue engineering. Polym. Bull. 77, 5985–6003 (2020). https://doi.org/10.1007/s00289-019-03057-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03057-7