Abstract
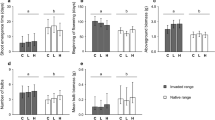
Invasion success is favoured by the introduction of pre-adapted genotypes. In addition, novel pressures in the introduced range may lead to phenotypic changes related to fitness or competitive ability of introduced plants. Polyploidy appears to be over-represented in invasive plants, but differences between cytotypes in growth strategies including trade-offs among plant traits have received little attention so far in the context of biological invasions. We grew Centaurea stoebe L. and Senecio inaequidens D.C. in a greenhouse experiment to test for differences in fitness (shoot biomass, reproductive output) and competitive ability (vegetative size, specific leaf area, leaf dry matter content, root–shoot ratio) between diploid and polyploid cytotypes as well as between native and introduced plants. For both species, diploid and tetraploid genotypes occur in the native range, whereas only tetraploids are present in the introduced range. In the native range of both species, diploid and tetraploid genotypes had different growth strategies. Tetraploid genotypes of C. stoebe and S. inaequidens had, respectively, higher specific leaf area and stem height than diploid ones. Thus, for both species, native tetraploids appeared more competitive than native diploids, which could explain, at least partially, the invasion success of the pre-adapted tetraploid genotypes. The comparison of native and introduced tetraploid genotypes revealed differences in traits linked to competitive ability, which could be linked to novel selection in the new environment. In S. inaequidens, we found evidence for a competition-colonisation trade-off, whereas persistence of C. stoebe in the new range seemed to be linked to a competition-defence trade-off.



Similar content being viewed by others
References
Bornkamm R (2002) On the phytosociological affiliations of an invasive species Senecio inaequidens in Berlin. Preslia Praha 74:395–407
Bossdorf O, Lipowsky A, Prati D (2008) Selection of preadapted populations allowed Senecio inaequidens to invade Central Europe. Divers Distrib 14:676–685
Broennimann O, Treier UA, Muller-Scharer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Campobasso G, Sobhian R, Knutson L, Pastorino AC, Dunn PH (1994) Biology of Pterolonche inspersa (Lep, Pterolonchidae), a biological control agent for Centaurea diffusa and Centaurea maculosa in the United States. Entomophaga 39:377–384
Chun YJ, Collyer ML, Moloney KA, Nason JD (2007) Phenotypic plasticity of native vs. invasive purple loosestrife: a two-state multivariate approach. Ecology 88:1499–1512
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Coomes DA, Grubb PJ (2003) Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol Evol 18:283–291
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
Diaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, Montserrat-Marti G, Grime JP, Zarrinkamar F, Asri Y, Band SR, Basconcelo S, Castro-Diez P, Funes G, Hamzehee B, Khoshnevi M, Perez-Harguindeguy N, Perez-Rontome MC, Shirvany FA, Vendramini F, Yazdani S, Abbas-Azimi R, Bogaard A, Boustani S, Charles M, Dehghan M, de Torres-Espuny L, Falczuk V, Guerrero-Campo J, Hynd A, Jones G, Kowsary E, Kazemi-Saeed F, Maestro-Martinez M, Romo-Diez A, Shaw S, Siavash B, Villar-Salvador P, Zak MR (2004) The plant traits that drive ecosystems: evidence from three continents. J Veg Sci 15:295–304
Dietz H, Edwards PJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367
Duncan C (2001) Knapweed management: another decade of changes. In: Smith L (ed) Proceedings of the First International Knapweed Symposium of the 21st Century. United States Department of Agriculture, Agricultural Research Service, Albany, CA, USA. Coeur d’Alene, Idaho, pp 1–7
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Colloquium on Variation and Evolution in Plants and Micro-organisms—toward a new synthesis 50 years after Stebbins. National Academy of Science, Irvine, pp 7043–7050
Ernst WHO (1998) Invasion, dispersal and ecology of the South African neophyte Senecio inaequidens in The Netherlands: from wool alien to railway and road alien. Acta Botanica Neerlandica 47:131–151
Facon B, Genton BJ, Shykoff J, Jarne P, Estoup A, David P (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21:130–135
Goldberg DE, Landa K (1991) Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J Ecol 79:1013–1030
Grime JP (1977) Evidence for existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Guesewell S, Jakobs G, Weber E (2006) Native and introduced populations of Solidago gigantea differ in shoot production but not in leaf traits or litter decomposition. Funct Ecol 20:575–584
Henery ML, Bowman G, Mráz P, Treier UA, Gex-Fabry E, Schaffner U, Müller-Schärer H (2010) Evidence for a combination of pre-adapted traits and rapid adaptive change in the invasive plant Centaurea stoebe. J Ecol 98:800–813
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15
Hook PB, Olson BE, Wraith JM (2004) Effects of the invasive forb Centaurea maculosa on grassland carbon and nitrogen pools in Montana, USA. Ecosystems 7:686–694
Hufbauer RA, Torchin ME (2007) Integrating ecological and evolutionary theory of biological invasions. In: Nentwig W (ed) Biological invasions. Springer, Berlin, Heidelberg, pp 79–96
Keddy P, Fraser LH, Wisheu IC (1998) A comparative approach to examine competitive response of 48 wetland plant species. J Veget Sci 9:777–786
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kuster EC, Kuhn I, Bruelheide H, Klotz S (2008) Trait interactions help explain plant invasion success in the German flora. J Ecol 96:860–868
Lafuma L, Balkwill K, Imbert E, Verlaque R, Maurice S (2003) Ploidy level and origin of the European invasive weed Senecio inaequidens (Asteraceae). Plant Syst Evol 243:59–72
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Muth NZ, Pigliucci M (2006) Traits of invasives reconsidered: Phenotypic comparisons of introduced invasive and introduced noninvasive plant species within two closely related clades. Am J Bot 93:188–196
Navas ML, Moreau-Richard J (2005) Can traits predict the competitive response of herbaceous Mediterranean species? Acta Oecol 27:107–114
Petit C, Thompson JD (1999) Species diversity and ecological range in relation to ploidy level in the flora of the Pyrenees. Evol Ecol 13:45–66
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65
Pinheiro JC, Bates DM (2000) Mixed-effects model in S and S-plus. Springer, New York
Poorter H, De Jong R (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol 143:163–176
Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ (2008) Adaptive evolution in invasive species. Trends Plant Sci 13:288–294
Prieur-Richard AH, Lavorel S, Grigulis K, Dos Santos A (2000) Plant community diversity and invisibility by exotics: invasion of Mediterranean old fields by Conyza bonariensis and Conyza canadensis. Ecol Lett 3:412–422
Pyšek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions. Springer, Berlin
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: A test across six biomes. Ecology 80:1955–1969
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Richardson DM, Pysek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611
Skinner K, Smith L, Rice P (2000) Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci 48:640–644
Soltis PS, Soltis DE (2000) The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci USA 97:7051–7057
Suding KN, Goldberg DE, Hartman KM (2003) Relationships among species traits: separating levels of response and identifying linkages to abundance. Ecology 84:1–16
Treier UA, Broennimann O, Normand S, Guisan A, Schaffner U, Steinger T, Müller-Schärer H (2009) Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90:1366–1377
Turnbull LA, Coomes D, Hector A, Rees M (2004) Seed mass and the competition/colonization trade-off: competitive interactions and spatial patterns in a guild of annual plants. J Ecol 92:97–109
Venables WN, Ripley BD (1999) Modern applied statistics with S-Plus. Springer-Verlag, New-York
Von Holle B (2005) Biotic resistance to invader establishment of a southern Appalachian plant community is determined by environmental conditions. J Ecol 93:16–26
Watson AK, Renney AJ (1974) The biology of Canadian weeds: Centaurea diffusa and Centaurea maculosa. Can J Plant Sci 54:687–701
Weber E, Schmid B (1998) Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am J Bot 85:1110–1121
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging theophrastus: a common core list of plant traits for functional ecology. J Veget Science 10:609–620
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227
Whitney KD, Gabler CA (2008) Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Divers Distrib 14:569–580
Williamson M (1996) Biological invasions. Chapman and Hall, London
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Acknowledgments
This project was funded by the National Centre of Competence in Research (NCCR) Plant Survival, research programme of the Swiss National Science Foundation. We thank Joanne Félix, Elena Rossel and Damien Pasche for technical assistance. We are grateful to Olivier Broennimann, Signe Normand and Urs Treier for collecting seeds of Centaurea stoebe, and to Daniel Prati and Isabelle Olivieri for providing seeds of Senecio inaequidens. We also thank the University of Lausanne for providing greenhouse facilities and material. This paper benefited from discussions and comments by Sandra Lavorel, Séverine Vuilleumier and Pierre Liancourt. The authors are grateful to two anonymous reviewers whose comments greatly improved the manuscript. This experiment complied with the Swiss regulation for invasive plant experimentations, as stated by the Federal Office for the Environment (FOEN).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thébault, A., Gillet, F., Müller-Schärer, H. et al. Polyploidy and invasion success: trait trade-offs in native and introduced cytotypes of two Asteraceae species. Plant Ecol 212, 315–325 (2011). https://doi.org/10.1007/s11258-010-9824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-010-9824-8