Abstract
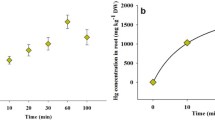
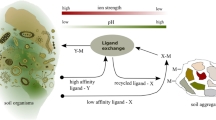
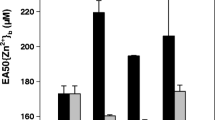
Commonly used equilibrium models for metal biouptake, such as the Free Ion Activity Model (FIAM) and the Biotic Ligand Model (BLM), are limited to the cases in which mass diffusive transport is not the flux-determining step. In analyses of metal biouptake from a complexing medium, all the physical (diffusion), chemical (dissociation kinetics of metal complexes), and biological (transport and internalization) processes have to be taken into account. A short-term zinc uptake by wheat (Triticum aestivum) roots from culture solutions in the absence or presence of synthetic ligands (NTA, nitrilotriacetic acid, and EDTA, ethylenediaminetetraacetate) was studied. At the same free Zn2+ concentration \(\left( {\left\{ {{\text{Zn}}^{{\text{2 + }}} } \right\} = 1.5 \times 10^{ - 8} {\text{M}}} \right)\) , the uptake of Zn was significantly enhanced in the presence of ligands and was larger when Zn complexes have a quicker dissociation rate. The diffusional fluxes in the same culture solution were determined with the differential pulse anodic stripping voltammetry (DPASV) method, and the diffusive gels in thin film (DGT) technique. The contribution from Zn complexes to root Zn uptake was in better agreement with the degree of Zn complex labilities measured with DPASV than with DGT. The diffusion of free Zn2+ ion to the root surface is a rate-controlling step for Zinc biouptake when the free Zn2+ concentration is low. Based on the comprehensive consideration of the diffusion and dissociation processes of Zn2+ ion and Zn complexes and the existence of high- and low-affinity uptake systems in the root surface, a two-pathway Zn uptake model was developed to predict the resulting Zn uptake fluxes into roots in the overall range of exposure.





Similar content being viewed by others
Abbreviations
- FIAM:
-
free ion activity model
- BLM:
-
biotic ligand model
- DPASV:
-
differential pulse anodic stripping voltammetry
- DGT:
-
diffusive gels in thin film
- SPUB-Model:
-
singular pathway uptake Best equation model
- TPUB-Model:
-
two-pathway uptake Best equation model
- RFW:
-
root fresh weight
- K M,i :
-
the characteristic bioaffinity parameter of roots
- \(J_{u,i}^* \) :
-
limiting uptake flux of roots
- \(c_M^0 \) and \(c_M^* \):
-
the concentration of the species M at the biosurface and in the bulk solution, respectively
- ξ :
-
the degree of metal complex lability
- ka and kd:
-
the association and dissociation rate, respectively
- K :
-
the conditional stability constant of complexation equilibrium
- DM and DML :
-
the diffusion coefficients of the free metal M and complex ML, respectively
- δ M :
-
the steady-state diffusion layer thickness
- µ :
-
the reaction layer thickness
References
Allen HE, Hall RH, Brisbin TD (1980) Metal speciation: Effects on aquatic toxicity. Environ Sci Technol 14:441–443. doi:10.1021/es60164a002
Antunes PMC (2004) Beyond the free ion activity model: the biotic ligand model for estimating copper uptake by durum wheat. PhD thesis. University of Guelph, ON, Canada
Antunes PMC, Hale BA (2006) The effect of metal diffusion and supply limitations on conditional stability constants determined for durum wheat roots. Plant Soil 284:229–241. doi:10.1007/s11104-006-0035-y
Bell PF, Chaney RL, Angle JS (1991) Free metal activity and total metal concentrations of micronutrient availability to barley (Hordeum vulgare (L.) ‘Klages’). Plant Soil 130:51–62. doi:10.1007/BF00011855
Bell PF, McLaughlin MJ, Cozens G, Stevens DP, Owens G, South H (2003) Plant uptake of 14C-EDTA, 14C-Citrate, and 14C-Histidine from chelator–buffered and conventional hydroponic solutions. Plant Soil 253:311–319. doi:10.1023/A:1024836032584
Berkelaar E, Hale B (2000) The relationship between root morphology and cadmium accumulation in seedling of two durum wheat cultivars. Can J Bot 78:381–387. doi:10.1139/cjb-78-3-381
Best JB (1955) The inference of intracellular enzymatic properties from kinetic data obtained on living cells. I. Some kinetic considerations regarding an enzyme enclosed by a diffusion barrier. J Cell Physiol 46:1–27. doi:10.1002/jcp.1030460102
Bosma TNP, Middeldorp PJM, Schraa G, Zehnder AJB (1997) Mass transfer limitation of biotransformation: Quantifying bioavailability. Environ Sci Technol 31:248–252. doi:10.1021/es960383u
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: A critical of the free ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems, Vol 3. John Wiley, New York, NY, USA, pp 45–10
Campbell PGC, Errecalde O, Fortin C, Hiriart-Baer VP, Vigneault B (2002) Metal bioavailability to phytoplankton–applicability of the biotic ligand model. Comp Biochem Physiol 133(Part C):189–206
Collins RN, Merrington G, Mclanghlin MJ, Mclanghine MJ, Knudsen C (2002) Uptake of intact Zn-ethlenediaminetetraacetic acid from soil is dependent on plant species and complex concentration. Environ Toxicol Chem 21:1940–1945. doi:10.1897/1551-5028(2002)021<:1940:UOIZEA>2.0.CO;2
Crank J (1964) The mathematics of diffusion. Oxford University Press, Toronto
Davison W, Zhang H (1994) In situ speciation measurements of trace components in nature waters using thin-film gels. Nature 367:546–548. doi:10.1038/367546a0
Degryse F, Smolders E, Merckx R (2006a) Labile Cd complexes increase Cd availability to plants. Environ Sci Technol 40:830–836. doi:10.1021/es050894t
Degryse F, Smolders E, Parker DR (2006b) Metal complexes increase uptake of Zn and Cu by plants: Implications for uptake and deficiency studies in chelator–buffered solutions. Plant Soil 289:171–185. doi:10.1007/s11104-006-9121-4
Eide DJ (1997) Molecular biology of iron and zinc uptake in eukaryotes. Curr Opin Cell Biol 9:573–577. doi:10.1016/S0955-0674(97)80036-1
Eigen M (1965) Fast elementary steps in chemical reaction mechanism. Pure Appl Chem 6:97
Florence TM (1986) Electrochemical approaches to trace element speciation in waters: a review. Analyst (Lond) 111:489–505. doi:10.1039/an9861100489
Hacisalihoglu G, Hart JJ, Kochian LV (2001) High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol 25:456–463. doi:10.1104/pp.125.1.456
Hassler CS, Wilkinson KJ (2003) Failure of the biotic ligand and free-ion activity models to explain zinc bioaccumulation by Chlorella kesslerii. Environ Toxicol Chem 22:620–626. doi:10.1897/1551-5028(2003)022<0620:FOTBLA>2.0.CO;2
Hassler CS, Slaveykova VL, Wilkinson KJ (2004) Some fundamental (and often overlooked) considerations underlying the free ion activity and biotic ligand models. Environ Toxicol Chem 23:283–291. doi:10.1897/03-149
Hering JG, Morel MM (1990) The kinetics of trace metal complexation: Implications for metal reactivity in natural waters. In: Stumm W (ed) Aquatic chemical kinetics: reaction rates of processes in natural waters. Environmental science and technology. Wiley, New York, pp 145–171
Hudson RJM (1998) Which aqueous species control the rates of trace metal uptake by aquatic biotic? Observations and predictions of non-equilibrium effects. Sci Total Environ 219:95–115. doi:10.1016/S0048-9697(98)00230-7
Jansen S, Blust R, Van Leeuwen HP (2002) Metal speciation dynamics and bioavailability: Zn (II) and Cd (II) uptake by Mussel (Mytilus edulis) and Carp (Cyprinus carpio). Environ Sci Technol 36:2164–2170. doi:10.1021/es010219t
Kariuki S, Dewald HD (1996) Evaluation of diffusion coefficients of metallic ions in aqueous solutions. Electroanalysis 8:307–312. doi:10.1002/elan.1140080402
Lavich VG (1962) Physicochemical hydrodynamics. Prentice-Hall, Englewood Cliffs, NJ, p 700
Lidon FC, Henriques FS (1992) Effects of increasing concentrations of Cu on metal uptake kinetics and biomass yields. Soil Sci 154:44–49
Luo XS, Zhou DM, Liu XH, Wang YJ (2006) Solid/solution partitioning and speciation of heavy metals in the contaminated agricultural soils around a copper mine in eastern Nanjing city, China. J Hazard Mater 131:19–27. doi:10.1016/j.jhazmat.2005.09.033
Martell AE, Smith RM, Motekaitis RJ (2001) NIST standard reference database 46 Version 6.0: NIST critically selected stability constants of metal complexes. NIST Standard Reference Data, Gaithersburg, MD
McLaughlin MJ, Smolders E, Merchx R, Maes A (1997) Plant uptake of Cd and Zn in chelator-buffered nutrient solution depends on ligands on ligand type. In: Ando T, Mori S (eds) Plant nutrition–for sustainable food production and environment. Kluwer Academic Publishers, Dordrecht, Netherlands
McLaughlin MJ, Andrew MJ, Smart MK, Smolders E (1998) Effects of sulfate on cadmium uptake by Swiss chard: I. Effects of complexation and calcium competition in nutrient solutions. Plant Soil 202:211–216. doi:10.1023/A:1004333529886
Morel FMM, Hering JG (1993) Principles and application of aquatic chemistry. Wiley, New York
Mylon SE, Twining BS, Fisher NS, Benoit G (2003) Relating the speciation of Cd, Cu, and Pb in two connecticut rivers with their uptake in algae. Environ Sci Technol 37:1261–1267. doi:10.1021/es0259281
Pinheiro JP, Van Leeuwen HP (2001) Metal speciation dynamics and bioavailability. 2. Radial diffusion effects in the microorganism range. Environ Sci Technol 35:894–900. doi:10.1021/es000042n
Pinheiro JP, Galceran J, Van Leeuwen HP (2004) Metal speciation dynamics and bioavailability: bulk depletion effects. Environ Sci Technol 38:2397–2405. doi:10.1021/es034579n
Sauvé S, Norvell WA, McBride M, Hendershot W (2000) Speciation and complexation of cadmium in extracted soil solutions. Environ Sci Technol 34:291–296. doi:10.1021/es990202z
Scally S, Davison W, Zhang H (2003) In situ measurements of dissociation kinetics and labilities of metal complexes in solution using DGT. Environ Sci Technol 37:1379–1384. doi:10.1021/es0202006
Slaveykova VI, Wilkinson KJ (2005) Predicting the bioavailability of metals and metal complexes: Critical review of the biotic ligand model. Environ Chem 2:9–24 doi:10.1071/EN04076
Sunda WG, Huntsman SA (1992) Feedback interactions between zinc and phytoplankton in seawater. Limnol Oceanogr 37:25–40
Tusseau-Vuillemin MH, Gilbin R, Taillefert M (2003) A dynamic numerical model to characterize labile metal complexes collected with diffusion gradient in thin films devices. Environ Sci Technol 37:1645–1652. doi:10.1021/es025839o
Unsworth ER, Warnken KW, Zhang H, Davison W, Black F, Buffle J et al (2006) Model predictions of metal speciation in freshwaters compared to measurements by in situ technique. Environ Sci Technol 40:1942–1949. doi:10.1021/es051246c
Van Leeuwen HP (1999) Metal speciation dynamics and bioavailability: Inert and labile complexes. Environ Sci Technol 33:3743–3748. doi:10.1021/es990362a
Van Leeuwen HP, Town RM, Buffle J, Cleven FMJ, Davison J, Puy J et al (2005) Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ Sci Technol 39:8545–8553. doi:10.1021/es050404x
Vassil AD, Kapulnik Y, Raskin I, Salt DE (1998) The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol 117:447–453. doi:10.1104/pp.117.2.447
von Wiren N, Marschner H, Romheld V (1996) Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol 111:1119–1125
Warnken KW, Zhang H, Davison W (2006) Accuracy of the diffusive gradients in thin-films technique: diffusive boundary layer and effectives sampling area considerations. Anal Chem 78:3780–3787. doi:10.1021/ac060139d
Wilkins RG (1991) Kinetics and mechanisms of reactions of transition metal complexes. Weinheim press, New York
Zhang H, Davison W (2000) Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using DGT. Anal Chim Acta 72:4447–4457
Acknowledgements
This work is financially supported by the CAS Research Program on Soil Biosystems and Agro-Product Safety (No. CXTD-Z2005–4–1) and the Knowledge Innovative Program of Chinese Academy of Sciences (No. KXCX3-SW-435). We thank Professor Hailin Zhang (Oklahoma State University, USA) for his suggestions and careful revisions on the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Appendix
Appendix
Establishment of the TPUB-Model for Zn uptake
The purpose of this appendix is to derive the mathematical expression of wheat root Zn uptake model. Firstly, we shall consider the situation in which an organism is in contact with the medium, containing a bioactive free metal ion M and bioinactive metal complex ML (charges have been omitted for simplicity).

where the association and dissociation rate are k a and k d, respectively. Under conditions of complexation equilibrium, the stability constant, K, can be described as:
where \({\text{C}}_{\text{i}}^* \) denotes the concentration of species i in the bulk solution.
For a singular pathway root uptake, the steady-state biouptake flux is modeled by the Best equation, which couples interfacial Michealis–Menten (MM) kinetics with a limiting flux of mass transfer towards the biosurface (Van Leeuwen 1999). For the medium with a bioactive metal ion M, bioinactive species ML, and a planar biosurface (e.g., higher plant roots), the biouptake step can be represented by a Michealis–Menten type of flux equation:
where J u is the actual metal uptake flux, \({\text{J}}_{\text{u}}^{\text{*}} \) is the limiting uptake flux, \({\text{c}}_{\text{M}}^{\text{0}} \) is the concentration of the bioactive species M at the biosurface, and K M is the characteristic bioaffinity parameters (equal to \({\text{c}}_{\text{M}}^{\text{0}} \) for Ju = 1/2Ju *). Mechanistically, the MM equation type steady-state flux describes a fast Langmuirian adsorption of the free metal M at the outer cell membrane followed by a first-order rate-limiting internalization step. For this uptake model, the analysis will concentrate on the \({\text{c}}_{\text{M}}^{\text{0}} \) and the supply of M from the complex medium with bulk concentrations \({\text{c}}_{\text{M}}^{\text{*}} \) and \({\text{c}}_{{\text{ML}}}^{\text{*}} \), coupled the reaction dynamics (M and ML interconversion rates governed by k a and k d) with diffusion process (diffusion rates governed by the diffusion coefficient DM of the free metal M and the diffusion coefficient DML of the complex ML).
The diffusional transport of metal in the medium can simply be written as the flux Jdiff that only related to M:
where \({\text{J}}_{{\text{diff}}}^{\text{*}} \) is the limiting diffusion flux for the case of \({\text{c}}_{\text{M}}^{\text{0}} = 0\,\left( {{\text{i}}{\text{.e}}{\text{.,}}\,{{{\text{D}}_{\text{M}} {\text{c}}_{_{\text{M}} }^* } \mathord{\left/ {\vphantom {{{\text{D}}_{\text{M}} {\text{c}}_{_{\text{M}} }^* } {\delta _{\text{M}} }}} \right. \kern-\nulldelimiterspace} {\delta _{\text{M}} }}} \right)\), δM is the steady-state diffusion layer thickness.
For wheat roots, there is a cylindrical diffusion formula (Crank 1964) for calculating the diffusional flux of the free ion M towards the roots.
where rroot is the radius of root and Δ is the width of the boundary layer at the root surface. Here,
Inclusion of Eq. 13 or 14 into Eq. 12 to eliminate \({\text{c}}_{\text{M}}^{\text{0}} \) yields a resulting biouptake flux expression model in terms of two most elementary parameters.
where Q is known as the Best equation (Bosma et al. 1997), “a” is the relative bioaffinity parameter, and “b” is the limiting flux ration. Low “a” values (a≪1) correspond to a high affinity of the organism for uptake, and large “b” values correspond to limiting diffusional transport conditions, in which the metal diffusion flux towards the root is lower than the biouptake flux. The ratio between “a” and “b” is the famous bioavailability number Bn (Bosma et al. 1997).
When the characteristic lifetimes of M and ML in reaction (Eigen 1965) are much shorter than the effective time scale of the experimental conditions (dynamic condition: k acL *t and k dt ≫1), and the kinetic flux of M resulting from net dissociation of ML (Jkin) is much greater than the purely diffusional flux of ML (Jdiff) (Van Leeuwen 1999), the complex ML is then said to be labile. Thus, the resulting diffusion flux of the free metal M can be written as (Degryse et al. 2006b):
where μ is the reaction layer thickness, a well-known concept in electrochemistry, which is a thin layer adjacent to the electrode surface, or in this case the root surface, in which the rate of dissociation of the metal complex is not fast enough to follow the depletion of the free metal ion (Van Leeuwen et al. 2005). The thickness of this reaction layer can be seen from Eq. 20
The combination of Eqs. 12 and 19 results in a resulting biouptake flux expression, in the form identical to Eq. 16
where
Based on the principle above and considering wheat roots with two different affinity uptake systems in the complex medium, we put forward a two-pathway uptake Best equation model (TPUB-Model). Here, the two pathways uptake MM equation can be written as:
where K M,1 and K M,2 denote the characteristic high- and low-affinity parameters, respectively; \({\text{J}}_{{\text{u,1}}}^{\text{*}} \) and \({\text{J}}_{{\text{u,2}}}^{\text{*}} \) denote, respectively, the limiting uptake fluxes of high- and low-affinity uptake systems. The diffusional flux expression of the free metal ion is the same as the above diffusional fluxes. The combination of the Eq. 1 with the diffusional flux expression yields a two-pathway uptake resulting flux expression: (we divided \({\text{c}}_{\text{M}}^{\text{*}} \) as \({\text{c}}_{\text{M}}^{\text{*}} \ll K_{{\text{M,2}}} \) and \({\text{c}}_{\text{M}}^{\text{*}} \gg 2K_{{\text{M,1}}} \) in order to easily resolve the equation by simplifying Eq. 1)
-
(1)
When \({\text{c}}_{\text{M}}^{\text{*}} \) is much smaller than K M,2 (i.e., \({\text{c}}_{\text{M}}^{\text{*}} > K_{{\text{M,2}}} \)), Eq. 1 can be simplified as:
$$J_u = J_{u\,,1}^* \frac{{c_M^0 }}{{K_{M\;,1} + c_M^0 }} + J_{u\,,2}^* \frac{{c_M^0 }}{{K_{M\,,2} }}$$(22)The two pathways uptake resulting flux expression can be seen as:
$$J_u = J_{u\,,2}^* \frac{{\left( {a_2 b_1 + a_1 b_2 + 2b_2 - a_1 a_2 - a_2 } \right)}}{{2_{b2} \left( {a_2 + b_2 } \right)}}\left\{ {1 - \left[ {1 - \frac{{4\left( {a_2 b_1 + a_1 b_2 + b_2 } \right)\left( {a_2 + b_2 } \right)}}{{\left( {a_2 b_1 + a_1 b_2 + 2b_2 - a_1 a_2 - a_2 } \right)^2 }}} \right]^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \right\}$$(2) -
(2)
When \({\text{c}}_{\text{M}}^{\text{*}} \) is larger than 2K M,1 (i.e., \({\text{c}}_{\text{M}}^{\text{*}} \ll 2K_{{\text{M,1}}} \)), Eq. 1 can be simplified as
$$J_u = J_{u\,,1}^* + J_{u\,,2}^* \frac{{c_M^0 }}{{K_{M\;,2} + c_M^0 }}$$(23)Therefore, the two-pathway uptake resulting flux expression can be written as:
$$J_u = J_{u\,,2}^* \frac{{\left( {b_1 + a_2 + b_2 + 1} \right)}}{{2b_2 }}\left\{ {1 - \left[ {1 - \frac{{4\left( {b_1 + b_2 + a_2 b_1 } \right)}}{{\left( {b_1 + a_2 + b_2 + 1} \right)^2 }}} \right]^{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \right\}$$(3)where a1, b1 and a2, b2 denote the a and b values of the high- and low-affinity uptake pathways, respectively, and their meaning is the same as defined above.
Rights and permissions
About this article
Cite this article
Wang, P., Zhou, D.M., Luo, X.S. et al. Effects of Zn-complexes on zinc uptake by wheat (Triticum aestivum) roots: a comprehensive consideration of physical, chemical and biological processes on biouptake. Plant Soil 316, 177–192 (2009). https://doi.org/10.1007/s11104-008-9769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9769-z