Abstract
Background and Aims
Projected changes in the atmospheric concentrations of CO2 and tropospheric O3 over the next 50 years are of significant concern due to the linkages in the cycling of carbon and water in forested ecosystems. Responses of tree roots to elevated CO2 (eCO2) and O3 (eO3) have been characterized primarily by studies of relatively shallow roots, yet deeper roots often play a disproportionately large role in water acquisition relative to their biomass. We undertook the present study to determine if there were significant root responses to eCO2 and eO3 below the maximum soil depths typically studied.
Methods
In the current study, we characterized small root biomass and morphometric responses to eCO2 and eO3 at the Aspen-FACE Experiment in Rhinelander, Wisconsin down to a depth of one meter.
Results
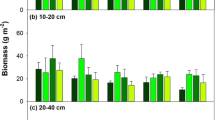
Elevated CO2 caused relatively undifferentiated growth stimulation. Elevated O3 stimulated root growth in the AA community at depth, while in the AB community there was a reduction in root growth in the shallow soil layer that was reversed in the deeper layers.
Conclusions
Root responses below depths typically studied were qualitatively similar than those within shallower soils for eCO2, but were sometimes compensatory for eO3.


Similar content being viewed by others
References
Afas N, Marron N, Zavalloni C, Ceulemans R (2008) Growth and production of a short-rotation coppice culture of poplar-IV: fine root characteristics of five poplar clones. Biomass and Bioenergy 32:494–502
Anderson C (2003) Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol 157:213–228
Bauhus J, Messier C (1998) Soil exploration strategies of fine roots in different tree species of the southern boreal forest of eastern Canada. Can J Forest Res 29:260–273
Bloom A, Chapin A, Mooney H (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Systemat 16:363–392
Bolte A, Villanueva I (2006) Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur J Forest Res 125:15–26
Brunner I, Godbold D (2007) Tree roots in a changing world. J Forest Res 12:78–82
Calfapietra C, Gielen C, Galema A, Lukac M, De Angelis P, Moscatelli M, Ceulemans R, Scarascia-Mugnozza G (2003) Free-air CO2 enrichment (FACE) enhances biomass production in a short-rotation poplar plantation. Tree Physiol 23:805–814
Ceulemans R, Mousseau M (1994) Tansley Review no. 71: Effects of elevated atmospheric CO2 on woody plants. New Phytol 127:425–446
Comas L, Eissenstat D (2004) Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct Ecol 18:388–397
Comas L, Bouma T, Eissenstat D (2002) Linking root traits to potential growth rate in six temperate tree species. Oecologia 132:34–43
Curtis P, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Dewar R, Franklin O, Mäkelä A, McMurtrie R, Valentine H (2009) Optimal function explains forest responses to global change. Bioscience 59:127–139
Dickson K, Lewin J, Isebrands M, Coleman W, Heilman D, Riemenschneider D, Sober,G, Host G, Zak G, Hendrey K, Pregitzer K, Karnosky D (2000) Forest Atmospheric Carbon Transfer and Storage (FACE-II) – The Aspen Free-air CO2 and O3 Enrichment (FACE) project: an overview. USDA Tech Rep NC-214, Washington DC.
Eissenstat D (1991) On the relationship between specific root length and rate of rot proliferation: a field study using citrus rootstocks. New Phytol 118:63–68
Eissenstat D (1997) Trade-offs in root form and function. In: Jackson L (ed) Ecology in agriculture. Academic, San Diego, pp 173–199
Eissenstat D, Wells C, Yanni R, Whitbeck J (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232:51–68
Franklin O, McMurtrie R, Iversen C, Crous K, Finzi A, Tissue D, Ellsworth D, Oren R, Norby R (2009) Forest fine-root production and nitrogen use under elevated CO2: contrasting responses in evergreen and deciduous trees explained by a common principle. Glob Chang Biol 15:132–144
Gebauer R, Reynolds J, Strain B (1996) Allometric relations and growth Pinus taeda: the effect of elevated CO2 and changing N availability. New Phytol 134:85–93
Hendrey G, Ellsworth D, Lewin K, Nagy J (1999) A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Glob Chang Biol 5:293–309
Hoosbeek M, Vos J, Bakker E, Scarascia-Mugnozza G (2006) Effects of free atmospheric CO2 enrichment (FACE), N fertilization and poplar genotype on the physical protection of carbon in the mineral soil of a polar plantation after 5 years. Biogeosciences 3:479–487
Huang B, Eissenstat D (2000) Linking root hydraulic conductivity to anatomy in citrus root stocks that vary in specific root length. Journal of the American Society of Horticultural Science 125:260–264
Iversen C (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357
Iversen C, Ledford J, Norby R (2008) CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol 179:837–847
Jackson R, Mooney H, Schulze E (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci 94:7362–7366
Jackson R, Cook C, Pippen J, Palmer S (2009) Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90:3352–3366
Karnosky D, Zak D, Pregitzer K, Awmack C, Bockheim J, Dickson R, Hendrey G, Host G, King J, Kopper B, Kruger E, Kubiske M, Lindroth R, Mattson W, McDonald NA, Oksanen E, Parsons W, Percy K, Podila G, Riemenschneider D, Sharma P, Thakur R, Sober A, Sober J, Jones W, Anttonen S, Vapaavuori E, Mankovska B, Heilman E, Isebrands J (2003) Tropospheric O3 moderates responses of temperate hardwood forests to eCO2: a synthesis of molecular to ecosystem results from the Aspen-FACE project. Functional Ecology 17:289–304
Kasurinen A, Kokko-Gonzales P, Riikonen J, Vapaavuori E, Holopainen T (2004) Soil CO2 efflux of two silver birch clones exposed to eCO2 and O3 levels during three growing seasons. Glob Chang Biol 10:1654–1665
King J, Thomas R, Strain B (1996) Growth and carbon accumulation in root systems of Pinus taeda and Pinus ponderosa seedlings as affected by varying CO2, temperature and nitrogen. Tree Physiol 16:635–642
King J, Pregitzer K, Zak D, Sober J, Isebrands J, Dickson R, Hendrey G, Karnosky D (2001) Fine-root biomass and fluxes of soil carbon in yound stands of paper birch and trembling aspen as affected by elevated atmospheric CO2 and tropospheric O3. Oecologia 128:237–250
King J, Kubiske M, Pregitzer K, Hendrey G, McDonald E, Giardina C, Quinn V, Karnosky D (2005) Tropospheric O3 compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2. New Phytol 168:623–636
Körner C (2006) Tansley review—plant CO2 responses: an issue of definition, time and resource supply. New Phytol 172:393–411
Kostiainen K, Kaakinen S, Warsta E, Kubiske M, Nelson N, Sober J, Karnosky D, Saranpää P, Vapaavuori E (2008) Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3. Tree Physiol 28:805–813
Kubiske M, Quinn V, Heilman W, McDonald E, Marquardt P, Teclaw R, Friend A, Karnosky D (2007) Interannual climatic version mediates elevated CO2 and O3 effects on forest growth. Glob Chang Biol 12:1054–1068
Kurz W, Kimmins P (1987) Analysis of some sources of error in methods used to determine fine root production in forest ecosystems: a simulation approach. Can J Forest Res 17:919–912
Lichter J, Barron S, Bevacqua C, Finzi A, Irving K, Stemmler E, Schlesinger W (2005) Soil carbon sequestration and turnover in a pine forest after 6 years of atmospheric CO2 enrichment. Ecology 86:1835–1847
López B, Sabaté S, Gracia C (2001) Fine-root longevity of Quercus ilex. New Phytol 151:437–441
López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Lukac M, Calfapietra C, Godbold D (2003) Production, turnover, and mycorrhizal colonization of three Populus species grown under eCO2 (Pop-FACE). Glob Chang Biol 9:838–848
Luo Y, Su B, Currie W, Dukes J, Finzi A, Hartwig U, Hungate B, Mcmurtrie R, Oren R, Parton W, Pataki D, Shaw M, Zak D, Field C (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
McCarthy M, Enquist B (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720
McMurtrie R, Norby R, Medlyn B, Dewar R, Pepper D, Reich P, Barton C (2008) Why is plant-growth response to eCO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimization hypothesis. Funct Plant Biol 35:521–534
Meinen C, Hertel D, Leuschner C (2009) Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: is there evidence of below-ground overyielding? Oecologia 161:99–111
Norby R, Iversen C (2006) Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology 87:5–14
Norby R, Wullschleger S, Gunderson C, Johnson D, Ceulemans R (1999) Tree responses to elevated CO2 in field experiments: implications for the future forests. Plant Cell Environ 22:683–714
Norby R, Ledford J, Reilly C, Miller N, O'Neill E (2004) Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci 101:9689–9693
Norby R, DeLucia E, Gielen B, Calfapietra C, Giardina C, King J, Ledford J, McCarthy H, Moore D, Ceulemans R, De Angelis P, Finzi A, Karnosky D, Kubiske M, Lukac M, Pregitzer K, Scarascia-Mugnozza G, Schlesinger W, Oren R (2005) Forest response to eCO2 is conserved across a broad range of productivity. Proc Natl Acad Sci 102:18052–18056
Oren R, Ewers B, Todd P, Phillips N, Katul G (1998) Water balance delineates the soil layer in which moisture affects canopy conductance. Ecol Appl 8:990–1002
Oren R, Ellsworth D, Johnsen K, Phillips N, Ewers B, Maier C, Schafer K, McCarthy H, Hendrey G, McNulty S, Katul G (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Percy K, Nosal M, Heilman W, Dann T, Sober J, Legge A, Karnosky D (2007) New exposure-based metric approach for evaluating O3 risk to North American aspen forests. Environ Pollut 147:554–566
Pregitzer K, Kubiske M, Yu C, Hendrick R (1997) Root architecture, carbon and nitrogen in four temperate forest species. Oecologia 111:302–308
Pregitzer K, Laskomski M, Burton A, Lessard V, Zak D (1998) Variation in northern hardwood root respiration with root diameter and soil depth. Tree Physiol 18:665–670
Pregitzer K, Zak D, Maziasz J, DeForest J, Curtis P, Lussenhop J (2000) Interactive effects of atmospheric CO2 and soil-N availability on fine roots of Populus tremuloides. Ecol Appl 10:18–33
Pregitzer K, DeForest J, Burton A, Allen M, Ruess R, Hendrick R (2002) Fine root architecture of nine North American trees. Ecol Monogr 72:293–309
Pregitzer K, Burton A, King J, Zak D (2008) Soil respiration, root biomass, and root turnover following long-term exposure of northern forests to elevated atmospheric CO2 and tropospheric O3. New Phytol 180:153–161
Prichard S, Strand A, McCormack M, Davis M, Finzi A, Jackson R, Matamala R, Rogers H, Oren R (2008) Fine root dynamics in a loblolly pine forest are influenced by free-air-CO2-enrichment: a six-year-minirhizotron study. Glob Chang Biol 14:1–15
Prior S, Rogers H, Runion G, Hendrey G (1994) Free-air CO2 enrichment of cotton: vertical and lateral root distribution patterns. Plant Soil 165:33–44
Publicover D, Vogt K (1993) A comparison of methods for estimating forest fine root production with respect to sources of error. Can J Forest Res 23:1179–1186
Reich P, Ellsworth D, Walters M (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reich P, Ellsworth D, Walters M (1997) From tropics to tundra: global convergence in plant functioning. Proc Nat Acad Sci 94:13730–13734
Reich P, Walters M, Tjoelker M, Vanderklein D, Buschene C (1998) Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct Ecol 12:395–405
Rhea L, King J, Kubiske M, Saliendra N, Teclaw R (2010) Effects of elevated atmospheric CO2 and tropospheric O3 on tree branch growth and implications for hydrologic budgeting. Environ Pollut 158:1079–1087
Rogers H, Runion G, Krupa S (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut 83:155–189
Ryser P (1996) The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Funct Ecol 10:717–723
Ryser P (1998) Intra- and interspecific variation in root length, root turnover and the underlying parameters. In: Lamberts H, Poorter H, Van Vuuren M (eds) Inherent variation in plant growth: physiological mechanisms and ecological consequences. Backhuys, Leiden, pp 441–465
Saxton A (1998) A macro for converting mean separation output to letter groupings in Proc Mixed. In Proc. 23 rd SAS Users Group Intl., SAS Institute, Cary, NC, pp 1243–1246
Schlesinger W, Lichter J (2001) Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2 . Nature: 466–469
Smucker A (1990) Quantification of root dynamics in agroecological systems. Rem Sens Rev 5:237–248
USDA Soil Conservation Service (1967) Soil survey and laboratory data and descriptions for some soils of Tennessee. Soil Survey Investigations Report No. 15, U.S. Dept. Agric., Soil Conservation Service and Tennessee Agricultural Experiment Station
Steinaker D, Wilson S (2005) Belowground litter contributions to nitrogen cycling at a northern grassland-forest boundary. Ecology 86:2825–2833
Stone E, Kalisz P (1991) On the maximum extent of tree roots. For Ecol Manage 46:59–102
Stoy P, Katul G, Siqueira M, e Juang J, Novick K, McCarthy H, Oishi A, Oren R (2008) Role of vegetation in determining carbon sequestration along ecological succession in the southeastern United States. Glob Chang Biol 14:1409–1427
Taylor H, Upchurch D, Brown J, Rogers H (1991) Some methods of root investigations. McMichael B, Persson H (eds.) Plant roots and their environment. Elsevier Science Publisher,. B.V., pp. 553-564
Taylor G, Tallis M, Giardina C, Percy K, Miglietta F, Gupta P, Gioli B, Calfapietra C, Gielen B, Kubiske M, Scarascia-Mugnozza G, Kets K, Long S, Karnosky D (2008) Future atmospheric CO2 leads to delayed autumnal scenescence. Glob Chang Biol 14:264–275
Uddling J, Teclaw R, Kubiske M, Pregitzer K, Ellsworth D (2008) Sap flux in pure aspen and mixed aspen-birch forests exposed to elevated concentrations of carbon dioxide and ozone. Tree Physiol 28:1231–1243
Uddling J, Teclaw R, Pregitzer K, Ellsworth D (2009) Leaf and canopy conductance in aspen and aspen-birch forests under free air enrichment of carbon dioxide and ozone. Tree Physiol 29:1367–1380
Vogt K, Vogt D, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Wells C (1999) Advances in the rot demography of woody species. PhD thesis, the Pennsylvania State University, USA
Wells C, Glenn D, Eissenstat D (2002) Changes in the risk of fine root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am J Bot 89:79–87
Acknowledgements
This work was conducted at the Aspen free air CO2 and O3 enrichment experiment at the USFS Northern Research Station Harshaw Experiment Station near Rhinelander, Wisconsin (Aspen FACE). Aspen FACE is principally supported by the U.S. Department of Energy’s Office of Biological and Environmental Research, Grant No. DE-FG02-95ER62125, to Michigan Technological University, and Contract No. DE-AC02-98CH10886 to Brookhaven National Laboratory, and the U.S. Forest Service. Major support specifically for this research at Aspen FACE was provided by USDA NRI Competitive Grants Program (2001-35107-11262 and 2004-35102-16723), USDA Forest Service, Northern Research Station, and the Department of Forestry and Environmental Sciences of the North Carolina State University.
The Department of Biology and the Plant and Vegetation Ecology Research Group (PLECO) of the University of Antwerp, the Belgian Francqui Foundation, and the U.S. Council for International Exchange of Scholars-Fulbright Program, provided sabbatical support to JSK during the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alain Pierret.
Rights and permissions
About this article
Cite this article
Rhea, L.K., King, J.S. Depth-dependency of trembling aspen and paper birch small-root responses to eCO2 and eO3 . Plant Soil 355, 215–229 (2012). https://doi.org/10.1007/s11104-011-1094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1094-2