Abstract
On the Hadean–Early Archean Earth, the first islands must have provided hot and dry environments for abiotically formed organic molecules. The heat sources, mainly volcanism and meteorite impacts, were also available on Mars during the Noachian period. In recent work simulating this scenario, we have shown that neat glycine forms a black, sparingly water-soluble polymer (“thermomelanoid”) when dry-heated at 200 °C under pure nitrogen. The present study explores whether relevant minerals and mineral mixtures can change this thermal behavior. Most experiments were conducted at 200 or 250 °C for 2 or 7 days. The mineral matrices used were phyllosilicates (Ca-montmorillonites SAz-1 and STx-1, Na-montmorillonite SAz-1-Na, nontronite NAu-1, kaolinite KGa-1), salts (NaCl, NaCl-KCl, CaCl2, artificial sea salt, gypsum, magnesite), picritic basalt, and three Martian regolith simulants (P-MRS, S-MRS, JSC Mars-1A). The main analytical method employed was high-performance liquid chromatography (HPLC). Glycine intercalated in SAz-1 and SAz-1-Na was well protected against thermomelanoid formation and sublimation at 200 °C: after 2 days, 95 and 79 %, respectively, had either survived unaltered or been transformed into the cyclic dipeptide (DKP) and linear peptides up to Gly6. The glycine survival rate followed the order SAz-1 > SAz-1-Na > STx-1 ≈ NAu-1 > KGa-1. Very good protection was also provided by artificial sea salt (84 % unaltered glycine after 200 °C for 7 days). P-MRS promoted the condensation up to Gly6, consistent with its high phyllosilicate content. The remaining matrices were less effective in preserving glycine as such or as peptides.
Similar content being viewed by others
Introduction
It is generally assumed that amino acids, the building blocks of proteins, were present on the young Earth through abiotic endogenous and exogenous sources. The simplest amino acid, glycine (H2NCH2COOH, Hgly), is among the most abundant amino acids in carbonaceous chondrites (Shock and Schulte 1990; Pizzarello et al. 2006; Zaia et al. 2008; Martins and Sephton 2009) and in the product mixtures obtained from Miller-type experiments (Miller 1953, 1955; Schlesinger and Miller 1983; Johnson et al. 2008). In general, peptide bond formation from amino acids is unfavorable in aqueous solution (Lambert 2008). The formation of peptides from glycine, however, is relatively easier because glycine is usually the most reactive amino acid in condensation reactions. In the solid state at higher temperatures, the methylene group of glycine can take part in an additional, “non-conventional” condensation process (Fox et al. 2015).
The prebiotic chemistry of glycine, as of any other prebiotic organic molecule, can only be adequately understood by considering the early Earth’s geochemical and geophysical conditions (Strasdeit 2010). Some experiments in this direction have already been conducted. In one example, in which volcanic or hydrothermal environments were simulated, the formation of diglycine (Gly2, Fig. 1) from glycine was observed in the presence of H2S (or CH3SH), CO and (Ni,Fe)S (Huber and Wächtershauser 1998). Later, it was shown experimentally and theoretically that glycine can be activated to glycine N-carboxyanhydride (Gly-NCA) by exposure to the volcanic gas COS (Leman et al. 2006; Nair et al. 2008). NCAs can form peptides and aminoacylated nucleotides, and they can activate inorganic phosphate (Biron and Pascal 2004; Leman et al. 2004, 2006; Biron et al. 2005). Under simulated conditions of submarine hydrothermal vents, oligomerization of glycine up to Gly10 was observed (Imai et al. 1999a, b; Tsukahara et al. 2002; Futamura et al. 2008). However, the prebiotic relevance of the conditions in such experiments has been questioned (Cleaves et al. 2009).
Evaporites and dissolved salts may have played an important role in the prebiotic chemistry of amino acids. It has been demonstrated, for example, that the heating of amino acids in artificial sea salt crusts produced alkylpyrroles, which are precursors of porphyrins (Fox and Strasdeit 2013). In the presence of anhydrous salts, the thermal formation of small homopeptides from glycine was observed (Kitadai et al. 2011). Glycine also forms peptides in relatively concentrated aqueous solutions of sodium chloride and copper (II) chloride (Schwendinger and Rode 1989; Rode and Schwendinger 1990). In these systems, sodium chloride serves as a dehydrating agent. It is assumed that various salts were already present on the Hadean Earth (Knauth 1998; Hazen 2013). Moreover, ancient salt deposits of chlorides, carbonates, and sulfates have been found on Mars (Rieder et al. 1997; Lellouch et al. 2000; Bandfield et al. 2003; Bibring et al. 2006; Wang et al. 2006; Fishbaugh et al. 2007; Noe Dobrea et al. 2008; Osterloo et al. 2008; Kuzmin et al. 2009; Palomba et al. 2009; Cloutis et al. 2010; Glotch et al. 2010; Morris et al. 2010; Wray et al. 2010).
In addition to salts, other inorganic compounds such as silica, alumina, and clay minerals can favor the condensation of glycine (see for example: Lahav et al. 1978; Lahav and White, 1980; Bujdák and Rode 1996, 1999; Georgelin et al. 2013; Fuchida et al. 2014; Martra et al. 2014). In the presence of these minerals, often the cyclic dipeptide (2,5-diketopiperazine DKP, Fig. 1) is formed as the main product. The possible importance of clay minerals in oligomerization and other prebiotic reactions was first suggested by Bernal (1949) and since then has been studied extensively (Ponnamperuma et al. 1982; Brack 2013). Clay minerals probably appeared very early in Earth’s history (Hazen 2013). Members of this mineral group, such as montmorillonites, nontronites, and kaolinite, were also identified on the Martian surface (Poulet et al. 2005, 2008; Mangold et al. 2007; Bishop et al. 2008; Mustard et al. 2008). There are a number of geological processes that produce clay minerals (Eberl 1984; Galán and Ferrell 2013). Among them is the weathering of volcanic rock and ash (Righi and Meunier 1995).
Volcanism was intense on the Hadean–Early Archean Earth (Nisbet and Fowler 2004; Taylor and McLennan 2009; Sleep 2010) and the Noachian Mars (Nimmo and Tanaka 2005; Xiao et al. 2012). Volcanoes probably protruded from the early Earth’s ocean (Buick and Dunlop 1990; Buick et al. 1995; Van Kranendonk et al. 2003, 2008; Westall et al. 2006; Taylor and McLennan 2009; Arndt and Nisbet 2012). They emitted dust particles and gases which could have protected subaerial surfaces from short-wavelength ultraviolet radiation and thus prevented organic molecules from decomposing (Westall et al. 2006). The same protective mechanisms may have been operative on Mars in its earlier epochs (Córdoba-Jabonero et al. 2003).
Volcanic heat and lightning have been discussed as sources of energy in prebiotic syntheses (Hill 1992; Basiuk and Navarro-González 1996; Navarro-González and Segura 2004). Another plausible energy source for the formation and destruction of organics is asteroid and comet impacts. Experimental impact simulations have been conducted, for example, with glycine. In these experiments, shock-induced decomposition of the amino acid has been observed (Bertrand et al. 2009; Sugahara and Mimura 2014a), but when deep-frozen targets were used, additionally oligoglycine formation up to the trimer occurred (Sugahara and Mimura 2014b). Stabilization and oligomerization of glycine were also observed in related high-pressure experiments simulating certain geological settings (Ohara et al. 2007; Otake et al. 2011).
Important episodes of prebiotic chemical and early biological evolution may have taken place on/at Hadean–Early Archean volcanic islands. There, for example, (i) various organics from endogenous production (e.g., volcanic lightning) and exogenous delivery as well as catalytically active minerals were probably available; (ii) light could have driven photosynthetic reactions; (iii) a wide range of temperatures existed, allowing different types of thermal reactions; (iv) the interaction between seawater and molten lava could have provided exceptional conditions for organic transformations (Fox and Strasdeit 2013); (v) wet–dry cycles could have occurred in smaller rock pools near the coasts. Larger rock pools could have had the size of a pond, and even lakes may have existed. They could have contained fresh water, if they were far enough inland. In fact, there are chemical arguments for an origin of life in a fresh-water environment (Deamer 2004).
Several of the minerals that are thought to have existed on primordial volcanic islands are known to interact with amino acids and other organic molecules. For example, it has been demonstrated that the clay mineral nontronite protects glycine against ultraviolet radiation (Poch et al. 2015). The role of minerals in the formation of oligoglycines has already been mentioned (see above).
Recently, we have shown that when neat glycine is heated to 200 °C, the main organic product is a black, sparingly water-soluble “thermomelanoid” (Fox et al. 2015). Is this reaction affected by the presence of minerals and rocks? In the present paper, we report the thermal properties of glycine in various inorganic matrices (clay minerals, salts and salt mixtures, basaltic volcanic rock, and Martian regolith simulants) and show that, in fact, some of these matrices drastically alter the thermal behavior of the amino acid. Some preliminary results have already been presented at international conferences (see for example: Dalai and Strasdeit 2009).
Materials and Methods
Chemicals, Minerals, Volcanic Rock, and Martian Regolith Simulants
The following chemicals were used without further purification: glycine (Acros, >99 %), 2,5-diketopiperazine (Fluka, 98 %), diglycine (Fluka, 99 %), triglycine, tetraglycine, pentaglycine, and hexaglycine (Sigma-Aldrich), sodium chloride (Merck, p.a.), potassium chloride (neoLab, p.a.), magnesium chloride hexahydrate (Merck, p.a.), calcium chloride dihydrate (Merck, p.a.), calcium sulfate dihydrate (Merck, >99 %), and magnesium carbonate (≥93 % MgCO3, Magnesia GmbH). Small amounts of dolomite and quartz were identified in the magnesium carbonate by X-ray powder diffraction. Double distilled water was prepared in a quartz glass distillation apparatus (BD 50, Westdeutsche Quarzschmelze) and was used throughout the study.
Ca-montmorillonites (SAz-1 and STx-1), nontronite (NAu-1), and kaolinite (KGa-1) were purchased from the Clay Minerals Society. These are particularly well characterized clay minerals (The Clay Minerals Society 2016). The Na-montmorillonite SAz-1-Na was prepared from Ca-montmorillonite SAz-1 by ion exchange (see below).
The volcanic rock used was an unweathered picrobasalt sand which had formed during the 2007 eruption of the Piton de la Fournaise volcano on the island of La Réunion, Indian Ocean. Within 2 weeks after collection in 2011, the sand was dried and sterilized at 150 °C for 24 h and then stored in a closed container at ~0 °C. Prior to use, the sand was pulverized. The major crystalline component was identified by X-ray powder diffraction as forsterite (Mg2SiO4), an end member of the olivine group [(Mg,Fe)2SiO4]. Elemental composition (determined by X-ray fluorescence spectrometry at the Landesamt für Geologie, Rohstoffe und Bergbau, Freiburg): SiO2 43.82, MgO 23.10, FeO 10.22, Al2O3 7.92, CaO 6.58, Fe2O3 3.05, TiO2 1.55, Na2O 1.55, K2O 0.38, MnO 0.18, P2O5 0.18 %; the remaining elements are mainly Cr (1.59 g kg−1) and Ni (0.94 g kg−1).
The “Phyllosilicatic Mars Regolith Simulant” (P-MRS) and “Sulfatic Mars Regolith Simulant” (S-MRS) used in this study were provided to us by J.-P. P. de Vera. They have been described in detail by Böttger et al. (2012). P-MRS and S-MRS have been prepared from igneous rocks, phyllosilicates, carbonates, sulfates, and iron oxides. The rocks and minerals are similar to those detected by Mars orbiter and rover missions (Bibring et al. 2005; Poulet et al. 2005; Chevrier and Mathé 2007; Morris et al. 2010) and in Martian meteorites (McSween 1994). The compositional differences between P-MRS and S-MRS reflect the environmental changes during Martian history. Another Martian regolith simulant, JSC Mars-1A, was purchased from Orbital Technologies Corporation (ORBITEC), Madison, Wisconsin. “JSC” refers to “NASA Johnson Space Center”. JSC Mars-1A is a weathering product of volcanic ash from Pu’u Nene, a cinder cone on the Island of Hawaii. It consists mainly of amorphous palagonite (Allen et al. 1997).
Prior to use, the picrobasalt and the Martian regolith simulants were stored in a desiccator over a saturated solution of Ca(NO3)2 for at least 2 days. This ensured a constant relative humidity (RH) of 51 % and thus defined water contents of the samples.
Synthetic Procedures
Preparation of ≤2 μm Clay Mineral Fractions
Clay mineral samples with particle sizes ≤2 μm were used throughout. To obtain this size fraction, the minerals from the Clay Minerals Society were first crushed to powders in a planetary ball mill (Retsch PM 100 at 100 rpm for 5 days, suspension in water, agate grinding jar with agate crushing balls). Afterwards, the suspensions were diluted with water, poured into Atterberg cylinders and left undisturbed for an appropriate settling time. The height of fall after the settling time was calculated for 2 μm sized particles. The part of the suspensions above this height, i.e., the ≤2 μm fraction, was isolated and centrifuged. The precipitates were dried, pulverized, and stored at 51 % RH. Settling times and heights of fall were calculated using the computer program “Atterberg” (Krumm 1994).
Preparation of Sodium Montmorillonite SAz-1-Na
This montmorillonite was synthesized by ion exchange from the Ca-montmorillonite SAz-1. For this, 15 g of wet SAz-1 with a particle size of ≤2 μm were mixed with 200 mL of a 2 mol L−1 NaCl solution. The suspension was shaken for 2 h and then centrifuged. In order to analyze the supernatant for dissolved calcium, a few drops of concentrated ammonia and an ammonium oxalate solution were added. Under these conditions, calcium ions, if present, precipitate as calcium oxalate. The treatment of the mineral with fresh NaCl solution was repeated until the calcium oxalate precipitation no longer occurred. Afterwards, the sample was washed chloride-free with water (tested with AgNO3/HNO3), dried, pulverized, and stored at 51 % RH. Vacuum-dried SAz-1-Na contained 2.89 % Na and 0.023 % Ca (determined by Mikroanalytisches Labor Pascher, Remagen, Germany). For comparison: the dried starting material SAz-1 contained 0.047 % Na and 2.25 % Ca (Mermut and Cano 2001). Thus, 99 % of the Ca2+ ions in SAz-1 had been replaced by Na+ by the ion exchange procedure. The Na and Ca content of SAz-1-Na corresponded to a cation exchange capacity of 127 meq/100 g. This value was in excellent agreement with the literature value of 123 ± 3 meq/100 g for SAz-1 (Borden and Giese 2001).
Loading of Clay Minerals, Volcanic Rock, and Martian Regolith Simulants with Glycine
Nine grams of Ca-montmorillonite, Na-montmorillonite or nontronite were suspended in 75 mL of a 0.5 mol L−1 aqueous glycine solution corresponding to 2.82 g of glycine. After shaking overnight, the suspension was centrifuged, and the supernatant was discarded. The resulting glycine-loaded mineral was manually pressed between two Whatman filter papers (grade 1573, 185 mm diameter) to remove residual glycine solution. The glycine-loaded clay minerals were then dried and pulverized. We call this procedure the “distribution method”, as it is based on the distribution of glycine between the solid and the liquid phase.
When loaded by use of the distribution method, SAz-1 contained 6.2 % glycine (see below). In order to obtain SAz-1 with a glycine content of exactly 1.0 %, “direct loading” was employed. In this method, 2.0 mg of glycine were dissolved in a small amount of water, and 198 mg of the mineral were added. The suspension was dried overnight at room temperature. A very small volume of water was used to wash glycine from the wall of the container back into the mineral. Then the suspension was dried again. The washing–drying procedure was repeated, typically twice, until the glycine was completely mixed with the mineral.
The direct loading method was also used for kaolinite because, compared to montmorillonite, this mineral shows a much lower equilibrium uptake of glycine from solution (Hedges and Hare 1987). A sample with an amino acid content of 5.0 % was obtained by loading 190 mg of kaolinite with 10 mg of glycine directly in the quartz container that was used for the subsequent thermolysis.
In the case of the picrobasalt and the Martian regolith simulants, 465 mg samples were loaded with 35 mg of glycine each, resulting in glycine contents of 7.0 %. The loading procedure was the direct loading described above.
Prior to use, all glycine-loaded samples were kept at 51 % RH for at least 2 days. This was necessary for a defined and reproducible water content. Otherwise the quantitative results could have been affected as the water content depends on the humidity, particularly for the smectites nontronite and montmorillonite, the latter also being present in P-MRS.
Preparation of Glycine-Containing Salt and Salt Mixtures
An NaCl-Hgly mixture was prepared from equimolar amounts of sodium chloride (1.75 g, 30 mmol) and glycine (2.25 g, 30 mmol). For an NaCl-KCl-Hgly mixture, 41.20 g (705 mmol) of sodium chloride, 1.12 g (15 mmol) of potassium chloride, and 0.75 g (10 mmol) of glycine were used. The components were completely dissolved in a sufficient amount of water, and the resulting solutions were evaporated in crystallizing dishes. The salt crusts formed were crushed and dried to constant weight in vacuo.
Similarly, a solution of calcium chloride dihydrate (14.70 g, 100 mmol) and glycine (7.51 g, 100 mmol) in 60 mL of water was evaporated on a rotary evaporator at 50 °C and finally dried to constant weight in vacuo. The infrared spectrum of this product was identical with that of the coordination compound CaCl2(Hgly) · H2O, which has been described by Yusenko et al. (2008).
An artificial sea salt-Hgly mixture was prepared by dissolving 41.20 g (705 mmol) of sodium chloride, 1.12 g (15 mmol) of potassium chloride, 16.26 g (80 mmol) of magnesium chloride hexahydrate, 2.21 g (15 mmol) of calcium chloride dihydrate, and 0.75 g (10 mmol) of glycine in 225 mL of water. The solution was evaporated in air. The remaining salt crust was crushed and vacuum dried to constant weight.
Calcium sulfate dihydrate (gypsum) dissolves slowly and has a relatively poor solubility in water. To obtain a gypsum-Hgly mixture, we therefore prepared the gypsum solution first. For this, 344 mg (2.0 mmol) of calcium sulfate dihydrate in 200 mL of water were shaken overnight. To the clear solution, 30 mg (0.40 mmol) of glycine were added. The solution was evaporated at room temperature. The remaining solid was collected and air-dried. It had a theoretical glycine content of 8.0 %.
To avoid hydrate and hydroxide formation and because magnesium carbonate (magnesite) has a very low solubility, a magnesite-Hgly mixture was prepared by dry mixing. Fine powders of magnesium carbonate (169 mg, 2.0 mmol) and glycine (30 mg, 0.40 mmol) were mixed and vigorously shaken. The resulting mixture had a theoretical glycine content of 15 %.
Thermal Treatment
All heating experiments were performed in a specially designed apparatus which has been described earlier (Fox and Strasdeit 2013). In brief, the central part of the apparatus consisted of a quartz glass tube (120 cm length, 4.0 cm inner diameter) placed in a tube furnace (Carbolite CTF 12/75/700). The sample container, which was also made of quartz glass, was initially positioned outside the heating zone. Prior to the experiment, the apparatus was purged with nitrogen gas of 99.999 % purity. Nitrogen simulated a non-oxidizing early atmosphere and, during the thermal treatment, also served as inert carrier gas to transport volatile products out of the furnace. The nitrogen flow was adjusted to 10 cm min−1 in the quartz glass tube. After 48 h of purging, the furnace was preheated to the desired temperature, and the sample container was pushed to the center of the heating zone. The nitrogen flow was maintained during the entire experiment.
Glycine-loaded clay minerals were heated at 200 or 250 °C for 2 days (48 h). In addition, the Ca-montmorillonites were also heated at 200 °C for 7 days (168 h). Sample amounts used were between 200 and 500 mg. Glycine-loaded picrobasalt and Martian regolith simulants (500 mg samples) were thermally treated at 200 °C for 2 days. Moreover, blank experiments were performed where 500 mg samples of unloaded SAz-1 and JSC Mars-1A were heated at 200 °C for 2 days.
NaCl-Hgly samples (400 mg each) were heated at 200 °C for 2 and 7 days. A 12 g sample of the NaCl-KCl-Hgly mixture was exposed to 200 °C for 7 days. Heating experiments with CaCl2(Hgly) · H2O were performed for 7 days with a 1.5 g sample at 200 °C and with 10-g samples at 230, 250, and 350 °C. Artificial sea salt-Hgly (3.5 g), gypsum-Hgly (200 mg), and magnesite-Hgly (200 mg) were thermally treated at 200 °C for 7 days.
The residues obtained after the thermal treatments were extracted with water, and the extracts were analyzed by high-performance liquid chromatography (HPLC, see below). Furthermore, infrared spectroscopy and X-ray powder diffractometry were applied to some of the residues.
Analytical Instrumentation
High-Performance Liquid Chromatography
The chromatograms were recorded with an HPLC system from Sykam (Fürstenfeldbruck, Germany). The system consisted of the reagent organizer S7121, solvent delivery system S1122, low pressure gradient mixer S8111, injector valve bracket S5111, and UV/Vis diode array detector S3210. The columns were placed in a column oven Jetstream II Plus (ERC, Riemerling, Germany).
Ion-pair chromatography: The column used was a Hypersil GOLD (3 μm particle size, 150 mm length, 4.6 mm internal diameter) from Thermo Fisher Scientific. The temperature of the column oven was 35 °C. An injection volume of 50 μL was used for quantification purposes. Detection was at 201 nm. The mobile phase consisted of 10 mmol L−1 sodium hexanesulfonate (C6H13SO3Na) in water acidified to pH 2.5 with phosphoric acid (Bujdák and Rode 1999). The flow rate was 1 mL min−1.
HPLC analysis of the 2,4-dinitrobenzene derivatives of glycine and the linear peptides: The derivatization was carried out as described by Bhushan and Brückner (2004) for the derivatization with Marfey’s reagent. The clay mineral extract (50 μL) was placed in a 2 mL plastic tube. One hundred microliters (3.5 μmol) of a 1 % solution of 1-fluoro-2,4-dinitrobenzene (Sanger’s reagent) and 20 μL (20 μmol) of 1 mol L−1 sodium hydrogen carbonate in water were added. After mixing, the tube was heated to 40 °C for 1 h with frequent mixing (every 5 to 10 min). After cooling to room temperature, 10 μL (20 μmol) of a 2 mol L−1 aqueous hydrochloric acid solution were added, and the resulting solution was mixed. After drying in a vacuum desiccator over sodium hydroxide, the residue was dissolved in 500 μL of dimethyl sulfoxide. The samples were protected from light. HPLC separation of the derivatives was performed on a Nucleodur C18 Gravity column (3 μm particle size, 150 mm length, 4.6 mm internal diameter) from Macherey-Nagel (Düren, Germany). The column oven temperature was 40 °C. An injection volume of 75 μl was used. Detection was at 340 nm. The mobile phase consisted of 10 mmol L−1 trifluoroacetic acid in water and acetonitrile. A linear gradient from 25 to 55 % acetonitrile within 20 min was used. After that, the concentration of acetonitrile was increased to 80 % within 15 min. The flow rate was 1 mL min−1.
Infrared Spectroscopy
The measurements were performed in attenuated total reflection (ATR) mode with a Thermo Nicolet 5700 FT-IR spectrometer equipped with an ATR Smart Orbit accessory. Measurement range: 4000–400 cm−1, number of scans per spectrum: 64, resolution: 4 cm−1.
MALDI–TOF/TOF Mass Spectrometry
Mass spectra were obtained on an Autoflex III instrument from Bruker Daltonics (Bremen, Germany). α-Cyano-4-hydroxycinnamic acid (CHCA) was used as matrix. To prepare the matrix solution, 5 mg of CHCA were dissolved in a mixture of 0.5 mL of water, 0.5 mL of acetonitrile, and 1 μL of trifluoroacetic acid. The analyte solution was mixed with the matrix solution in an appropriate ratio. Droplets of the resulting mixture were transferred onto the target plate and dried at room temperature.
High-Resolution/High-Accuracy Mass Spectrometry
A Thermo LTQ Orbitrap XL mass spectrometer (ThermoScientific, Bremen, Germany) equipped with an electrospray ionization (ESI) ion source was used. External mass calibration was performed according to the manufacturer’s guidelines using the Pierce LTQ ESI Positive Ion Calibration Solution (caffeine, MRFA and Ultramark 1621). For internal calibration, lock mass ions were used as described by Olsen et al. (2005). The spectrometer was operated in positive ion mode. An ionization voltage of 1.5 kV was used. The capillary temperature was 200 °C. The data were recorded in the 50 to 1800 Da mass range using the Orbitrap mass analyser which was operated with a target mass resolution of 60,000 (defined at m/z 400). The samples were introduced into the spectrometer through a BEH 130 C18 column (250 mm × 75 μm, 1.7 μm particle size; Waters, Milford, Massachusetts, USA) on a nanoACQUITY UPLC system (Waters, Milford, Massachusetts, USA). Gradient elution was performed from 1 to 50 % acetonitrile in 0.1 % aqueous formic acid within 60 min. The data were processed with Xcalibur software (version 2.0.7, ThermoScientific, Bremen, Germany).
X-Ray Powder Diffractometry
Diffractograms were recorded on a Bruker D8 Focus instrument equipped with a Sol-X energy dispersive detector. Data were measured with CuKα radiation (λ = 1.5418 Å) in the 2θ range 5–60° (5–80° for the picrobasalt). During the measurements, some samples (e.g., the ones containing smectites) were covered with polyimide foil to avoid changes in the water content.
Analytical Procedures
Glycine Content in Glycine-Loaded Clay Minerals
The amount of glycine in those clay mineral samples that had been prepared by the distribution method was determined by conductometric titration with sodium hydroxide solution. Additionally, HPLC quantification was performed (see below). The glycine contents determined by titration (T) and HPLC (H) were in good agreement with each other. The average of the two values was used as the basis for the calculation of yields after the thermal treatments. The glycine content of the Ca-montmorillonites was 6.2 % (T 6.3 %, H 6.1 %) in SAz-1 and 5.2 % (T 5.3 %, H 5.1 %) in STx-1. The glycine concentration in the sodium montmorillonite SAz-1-Na was 11.9 % (T 12.2 %, H 11.6 %) and thus about twice as large as in the Ca form. This value was confirmed by C and N elemental analysis (performed by Mikroanalytisches Labor Pascher, Remagen, Germany) which gave 4.08 % and 2.08 %, respectively, and from which a glycine content of 12.0 % was calculated. For the glycine-loaded nontronite, an amino acid content of 7.7 % (T 7.6 %, H 7.7 %) was determined, which is comparable to the values found in the Ca-montmorillonites. All values refer to samples that had been kept at 51 % RH for at least 2 days.
Extraction and Quantification of Glycine and Oligoglycines After Thermal Treatment of Glycine-Loaded Matrices
Prior to the extraction, the samples were kept at 51 % RH for at least 2 days. In total, 10 extraction stages were performed with each sample. For a 100 mg sample, the first three stages were performed with 600 μL of water each and and the next seven stages with 450 μL each. In each extraction stage, the sample suspension was shaken for 1 h and thereafter centrifuged. The supernatants were collected, and the final volume was made to 5 mL. This procedure resulted in a virtually complete extraction of glycine and oligoglycines. Prior to HPLC analysis, the extracts were again centrifuged to remove any residual solid particles. Glycine, DKP, diglycine, and triglycine were directly quantified by HPLC using calibration curves. The calibration curves were linear except for DKP. In order to also quantify the tetra-, penta- and hexapeptide in one of the experiments with SAz-1, glycine and the linear peptides were transformed into the 2,4-dinitrophenyl derivatives and then measured by HPLC. The absolute amounts of glycine and di- and tripeptide were known from measuring the underivatized compounds (see above). Therefore, the absolute amounts of the higher peptides could be calculated assuming that the extinction coefficient did not significantly vary between the different derivatives (for this assumption see for example: Fujio et al. 1959).
Results and Discussion
Recently, we have described a black organic polymer that was obtained by heating neat glycine under a nitrogen atmosphere (Fox et al. 2015). The formation of this “thermomelanoid” starts at ~160 °C and is complete at ~200 °C. When the temperature is raised further, the polymer progressively decomposes. The thermomelanoid is formed by condensation of glycine molecules via conventional amide bond formation and an additional condensation process involving C=O and CH2 groups (“hypercondensation”). Interestingly, glycine and diglycine are main hydrolysis products of the thermomelanoid in water. It can therefore be hypothesized that the thermomelanoid may have acted as a storage form of glycine and glycine peptides during prebiotic chemical evolution. Moreover, the low solubility of this polymer compared to the amino acid and its homopeptides may have influenced the distribution of glycine on the early Earth. In addition, the thermomelanoid is decomposed in soil, probably by microorganisms (currently unpublished work by Dalai (2013) in her doctoral thesis). Therefore, one might speculate that the thermomelanoid could have served as a nutrient source for Early Archean microorganisms, for example at the coasts of volcanic islands.
In the following sections, the influence of mineral matrices on the thermal behavior of glycine is described and discussed. The results are compared with the thermal properties of the neat amino acid. Starting point for our experiments was the well-known fact that many potentially prebiotic molecules interact with mineral surfaces and that these interactions may have been important for the origin of life (Hazen 2006; Cleaves et al. 2012).
Clay Minerals
Glycine-Loaded Calcium Montmorillonites SAz-1 and STx-1
A blank experiment was performed with unloaded SAz-1 at 200 °C for 2 days. No glycine or glycine peptides were detectable by HPLC in the water extract of the thermolysis residue. Thus, the presence of relevant amounts of these compounds could be excluded. Next, samples of glycine-loaded SAz-1 (6.2 % glycine content) were heated either at 200 °C or at 250 °C for 2 days. The subsequent qualitative HPLC analysis revealed that glycine had partly survived unreacted and partly formed DKP and linear peptides up to hexaglycine at both temperatures (Table 1 and Fig. 2). The peptides were identified by spiking with authentic samples. The MALDI–TOF/TOF mass spectrum was consistent with the presence of the peptides. However, most of them could not be unambiguously observed due to overlap with intense matrix peaks in the low-mass region. Therefore, high-resolution/high-accuracy mass spectrometry was performed. The measured masses were in excellent agreement with the calculated ones (Table 2). Thus, this method allowed the unambiguous identification of the peptides on the basis of their exact masses.
Glycine, DKP, and the linear peptides were also quantitatively determined by HPLC. In the 200 °C experiment, the glycine amount in the residue was 258 μmol. The starting material contained 413 μmol of glycine, meaning that 62 % of the amino acid had survived unreacted. Most of the remaining 155 μmol had been transformed into DKP (54 μmol), diglycine (11 μmol), triglycine (1 μmol), tetraglycine (0.3 μmol), pentaglycine (0.1 μmol), and hexaglycine (0.05 μmol). These values show that, at 200 °C, around 95 % of the glycine was still present or had been transformed into peptides. This is in sharp contrast to the outcome of 200 °C experiments with neat glycine, where the thermomelanoid is by far the major solid product.
To ascertain the influence of the heating time, a 200 °C experiment with glycine-loaded SAz-1 (6.2 % glycine content) was conducted for 7 days. The initial glycine amount in the sample was 165 μmol. At the end of the experiment, glycine (64 μmol), DKP (14 μmol), diglycine (5 μmol), and a small amount of triglycine but no longer peptides were detected by HPLC. Compared to the 2-day experiment at the same temperature, the survival rate of glycine was lower (39 % vs. 62 %). At the same time, the peptides formed tended to be shorter. Obviously, the longer peptides, which had been formed after 2 days, and some of the glycine decomposed during the additional reaction time. Cleaves et al. (2009) have made similar observations, even though their reaction conditions, simulating certain aspects of submarine hydrothermal vents, were very different from ours.
Although concentration dependence was not a central issue of this study, we performed an experiment with a sample of lower glycine content. In this experiment, SAz-1 loaded with 1.0 % of glycine was heated at 200 °C for 2 days. After the thermal treatment, the same compounds that had been obtained with a 6.2 % loaded sample under otherwise identical conditions were detected by HPLC, namely DKP, the linear peptides Gly2 to Gly6, and unreacted glycine. Peaks of unidentified decomposition products interfered with some of the peaks of interest so that only a qualitative discussion is possible. The main results are that (i) no peptides longer than the hexamer appeared in the chromatogram, (ii) DKP is the major peptide formed, and (iii) the amount of DKP relative to the other peptides appears to be lower than in the case of 6.2 % glycine loading. In conclusion, it can be said that going from 6.2 to 1.0 % glycine loading had rather small effects. Nevertheless, future studies on SAz-1 samples with much lower loadings may be worthwhile.
Heating of glycine-loaded SAz-1 at the higher temperature of 250 °C for 2 days gave significantly different results. While the amounts of diglycine (9 μmol) and triglycine (1 μmol) in the residue were comparable to those found in the 200 °C experiment, much more DKP (141 μmol) had formed, but much less glycine (29 μmol, 7 % of the initial amount) had survived. Thus, the higher temperature strongly favored the formation of the cyclic dipeptide: about 68 % of the starting amount of glycine were transformed into DKP. Mass losses of 14 and 15 % were measured in the 200 and 250 °C experiment, respectively. They were due to the escape of water from the interlayer spaces of the clay mineral and, to a lesser extent, to peptide bond formation. Some decomposition was obvious from unidentified peaks in the chromatograms (Fig. 2). Interestingly, no thermomelanoid formation and no sublimation were observed at both temperatures. We therefore conclude that the intercalation in the Ca-montmorillonite SAz-1 largely protects glycine against transformation into the thermomelanoid, sublimation, and extensive decomposition into non-peptide products.
In addition to SAz-1, we have used the Ca-montmorillonite STx-1 as a matrix for glycine. STx-1 differs from SAz-1 mainly by its lower content of interlayer cations, which is reflected in its lower cation exchange capacity of 89 ± 2 meq/100 g, compared to 123 ± 3 meq/100 g for SAz-1 (Borden and Giese 2001). The glycine loading obtained with STx-1 was 5.2 % and thus lower than in the case of SAz-1, despite the fact that the same method was used (distribution method, see Materials and Methods). Two samples of glycine-loaded STx-1, each containing 139 μmol of the amino acid, were exposed to 200 °C for 2 days and 7 days, respectively. After 2 days, the mineral contained 55 μmol of glycine (40 % of the initial amount) and 2 μmol of DKP and diglycine each. After 7 days, 43 μmol of glycine (31 % of the initial amount), 3 μmol of DKP, and 2 μmol of diglycine were found. Small amounts of triglycine had been formed in both experiments but could not be quantified. As in the experiments with SAz-1, peaks of unidentified decomposition products occurred. Interestingly, triglycine was the longest peptide identifiable by HPLC. The results showed that SAz-1 provided better protection to glycine than STx-1. Moreover, the yields of DKP and diglycine and the degree of polymerization of glycine were higher in SAz-1. It is unknown what exactly caused these differences, but they at least show that the “same” clay mineral may significantly differ in its effect on prebiotic organic molecules, depending on its provenance.
Glycine-Loaded Sodium Montmorillonite SAz-1-Na
The montmorillonite SAz-1-Na was prepared from the Ca-montmorillonite SAz-1 (see Materials and Methods). After loading, it contained 11.9 % of glycine, which was about twice the concentration found in loaded SAz-1 (6.2 %). It is well known that with Na+ as the exchangeable cation the interlayer swelling in water is higher than with Ca2+ (Odom 1984). Thus, during the loading process more water was displaced by glycine molecules, leading to a higher amino acid content of the Na-form. Similar results have been obtained by Benincasa et al. (2000). These authors also used the distribution method for loading SAz-1 and its Na-form, but their solution concentration of glycine was lower than ours (0.2 vs. 0.5 mol L−1). Consequently, they obtained somewhat lower loadings: 10.8 % for the Na-form and 3.6 % for the Ca-form (values read from Fig. 1a of Benincasa et al. (2000)).
We exposed our glycine-loaded SAz-1-Na to 200 °C for 2 days. Glycine, DKP, and linear peptides up to hexaglycine were detected after the thermal treatment. The survival rate of glycine was 53 % (168 μmol of initially 317 μmol). Twenty-six percent of the initial amount of glycine had been converted into DKP (39 μmol) and diglycine (2 μmol). The other glycine peptides were present in low amounts and were not quantified. After thermal treatment of glycine-loaded SAz-1-Na at the higher temperature of 250 °C for 2 days, the residue contained unreacted glycine (78 μmol, 25 % of the initial amount of 317 μmol) and DKP (62 μmol). A small, unquantified amount of diglycine was also detected, but higher peptides were absent. No sublimation was observed. Mass losses of 13 and 15 % occurred at 200 and 250 °C, respectively.
Comparison with SAz-1 (6.2 % loading) shows that at 200 °C the survival rate of glycine is higher in the Ca- than in the Na-form (62 vs. 53 %). Interestingly, at 250 °C the proportions reversed. At this temperature, the survival rate in the Ca-form was significantly lower (7 vs. 25 %). In both the Na- and the Ca-montmorillonite, the formation of DKP is favored at the higher temperature.
Glycine-Loaded Nontronite NAu-1
After exposure to 200 °C for 2 days, glycine, DKP, diglycine, and triglycine were present in the residue. The amount of glycine was 202 μmol, corresponding to 39 % of the initial 513 μmol. Thus, the percentage of unaltered glycine was almost the same as in the Ca-montmorillonite STx-1 but substantially lower than in the other two smectites (SAz-1 and its sodium form SAz-1-Na). Due to the lack of baseline separation, DKP could not be quantified. A small amount of diglycine (4 μmol) had formed. The amount of triglycine was very low and was not quantified. After heating of the glycine-loaded nontronite at 250 °C for 2 days, the residue still contained 127 μmol of glycine, which represented 25 % of the initial amount. DKP and diglycine could not be quantified as the peaks were not well resolved in the chromatograms, probably owing to interfering signals from unidentified decomposition products. No peptides higher than diglycine were observed.
Comparison of the different smectites shows that at 200 °C, the degree of polymerization was much lower in nontronite than in SAz-1 and SAz-1-Na but comparable to that found in STx-1. The amounts of other minerals (kaolin, goethite, quartz, and biotite) that are present in the ≤2 μm fraction of the nontronite (Keeling et al. 2000) are probably too small to influence its behavior. Hence, as in the case of STx-1, the relatively low tendency to protect and polymerize glycine is intrinsic to this mineral. Finally, mass losses of 10.5 and 16 % occurred at 200 and 250 °C, respectively, in the nontronite experiments.
Glycine-Loaded Kaolinite KGa-1
After exposure to 200 °C for 2 days, 15 % of the glycine (20 μmol of initially 133 μmol) had survived. DKP (15 μmol), diglycine (3 μmol), and triglycine (1 μmol), as well as small amounts of tetra- and pentaglycine, were identified by HPLC. Interestingly, Lahav et al. (1978) observed the same degree of polymerization in experiments where mixtures of glycine and a kaolinite were exposed to wetting–drying and temperatures fluctuation cycles. Residual glycine (8 μmol, 6 % of the initial amount), DKP (5 μmol), diglycine, and triglycine were also detected after heating glycine-loaded KGa-1 at 250 °C for 2 days. Di- and triglycine could not be quantified because of interfering peaks in the chromatogram. In a related study, Meng et al. (2007) reported that the linear dipeptide formed from kaolinite-adsorbed glycine in the temperature range 110–160 °C and further condensed to DKP at temperatures up to 210 °C.
It is remarkable that at both temperatures employed in our experiments a very thin layer of sublimate composed of glycine and DKP was found at the end of the quartz tube. This observation, which was not made for the other clay minerals studied, suggests that glycine was mainly adsorbed on the outer surfaces of the kaolinite particles and not intercalated. In fact, kaolinites intercalate only certain compounds such as formamide, dimethyl sulfoxide, and salts of short-chain fatty acids. The process of intercalation is slow, and usually temperatures above room temperature are needed (Lagaly et al. 2013). Hence, under our preparative conditions (see Materials and Methods), the intercalation of glycine seems unlikely. Kaolinite–glycine intercalates have been prepared, however, by displacing water from hydrated kaolinite, which in turn had been obtained from kaolinite–dimethyl sulfoxide or kaolinite–hydrazine intercalates (Sato 1999; Zheng et al. 2014). In contrast, intercalation into montmorillonites and nontronites is straightforward.
The results demonstrate that glycine can partly survive unreacted and partly condenses into peptides when heated at 200 or 250 °C in the presence of clay minerals (Table 1). When the montmorillonite SAz-1 was treated at 200 °C for 2 days, other processes such as complete decomposition, sublimation, and possibly thermomelanoid formation were less important, affecting only 5 % of the amino acid. These processes were, however, dominant in the case of the kaolinite KGa-1 where ~56 % were affected under the same experimental conditions. At 200 °C, the percentage of glycine that survived as such followed the order SAz-1 > SAz-1-Na > STx-1 ≈ NAu-1 > KGa-1. Moreover, the degree of polymerization was highest for SAz-1 and SAz-1-Na. Thus, at 200 °C, these two minerals provided the best protection for glycine. Coordination of the glycine molecules to the interlayer cations of the smectites may have promoted the polymerization. For instance, Remko and Rode (2001, 2004), in theoretical papers, reported that divalent metal cations facilitated the condensation of glycine to diglycine.
As may have been expected, the survival rate of glycine decreased when the temperature was raised to 250 °C. Except for SAz-1, the degree of polymerization also decreased at this higher temperature. At both temperatures, the cyclic dipeptide DKP was found to be the predominant condensation product in almost all experiments where quantification was possible. Only with STx-1 (2 days at 200 °C) equal amounts of DKP and Gly2 formed. In the cases of SAz-1 and SAz-1-Na, the predominance of DKP was even more apparent at the higher temperature: on going from 200 to 250 °C, the yield of DKP increased by a factor of 2.6 and 1.6, respectively. The results for SAz-1 allow to compare the effect of raising the temperature with that of a prolonged heating time. Interestingly, increasing the duration of the 200 °C experiment from 2 to 7 days was much less detrimental to the glycine survival rate than the temperature increase to 250 °C. The degree of polymerization and the yield of DKP, however, were both diminished by the extended reaction time, whereas the higher temperature caused no change and an increase, respectively.
Evaporites
NaCl-Glycine and NaCl-KCl-Glycine Mixtures
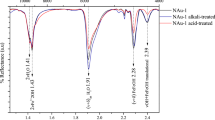
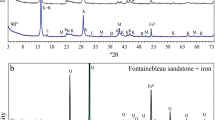
Evaporation of an aqueous solution of α-glycine and sodium chloride resulted in a crystalline mixture of the inorganic salt and the γ-modification of the amino acid. The infrared absorption bands of the NaCl-Hgly mixture were clearly shifted with respect to the positions for the initial α-glycine, sometimes by more than 10 cm−1 (Fig. 3, spectra A and B). They were in excellent agreement with those reported in the literature for γ-glycine (see for example: Ambujam et al. 2006). Additionally, comparison of the X-ray powder diffractogram of the NaCl-Hgly mixture with that of γ-glycine, which was calculated from single-crystal data (Kvick et al. 1980; Brandenburg 2015), unequivocally demonstrated the presence of the γ-form. Crystallization of the γ-polymorph in the presence of sodium chloride is a known phenomenon (see for example: Srinivasan and Arumugam 2007). Similarly, infrared spectra and X-ray powder diffractograms showed that the γ-form was also present in the NaCl-KCl-Hgly mixture.
Two aliquots of the NaCl-Hgly mixture (see Materials and Methods), each containing 3000 μmol of glycine, were heated at 200 °C for 2 and 7 days, respectively. At the end of the experiments, both samples had turned black. The color was caused by the thermomelanoid, which was identified by its infrared spectrum (Fig. 3, spectrum C) and X-ray powder diffractogram (Fox et al. 2015). HPLC analysis revealed that only 240 μmol (8 %) and 150 μmol (5 %) of the initial glycine had survived after 2 and 7 days, respectively. Additionally, 30 μmol of DKP were detected in each of the two residues, but no higher peptides were found. The mass loss observed after 2 days (21.5 %) corresponded to 38 % of the initial mass of glycine. The same value has been obtained when neat glycine was heated at 200 °C for 2 days (Fox et al. 2015). After 7 days, the mass loss of the NaCl-Hgly mixture was slightly higher (23.5 %), which corresponded to 42 % of the initial mass of the amino acid. The mass losses were mainly due to the formation of water of condensation and sublimation of DKP and glycine.
When the NaCl-KCl-Hgly mixture was exposed to 200 °C for 7 days, it became dark grey. As with NaCl-Hgly, infrared spectroscopy proved the formation of the thermomelanoid, and unreacted glycine and DKP could be detected by HPLC. The amount of glycine in the residue was determined to be 1200 μmol of initially 2780 μmol, whereas the amount of DKP was too low for quantification. In addition, a thin layer of sublimed glycine and DKP had deposited on the inner wall of the quartz glass tube outside the furnace. The percentage of glycine that had survived in the residue after 7 days was about 9 times higher in the NaCl-KCl matrix than in NaCl alone (Table 3). This was probably caused by the much higher salt–to–glycine molar ratio in NaCl-KCl-Hgly (72:1 compared to 1:1 in NaCl-Hgly), which might have provided a kind of mechanical protection to glycine.
Both the sodium and the potassium cation are known to form only weak complexes with neutral glycine molecules (Jover et al. 2008). Therefore, when NaCl-(KCl-)Hgly solutions are evaporated to dryness, the lattice energies of the metal chlorides dominate, and glycine remains uncoordinated, i.e., in its free form. This behavior contrasts that of calcium chloride (see below; Yusenko et al. 2008). The fact that glycine is not bonded to Na+ and K+ explains why solid NaCl-(KCl-)Hgly mixtures showed a thermal behavior similar to that of neat glycine, in particular a partial transformation into the thermomelanoid at 200 °C. On the other hand, our experiments also demonstrated that an NaCl(-KCl) matrix, at least when present in large excess, offered a certain protection against thermomelanoid formation, leaving some glycine unreacted. These salts are, however, ineffective in stabilizing linear oligopeptides. This is consistent with the results obtained with neat glycine, which showed that oligopeptides did not accumulate because they reacted further to form the polymeric thermomelanoid (Fox et al. 2015).
Glycine Coordinated to CaCl2
In another experiment, an aqueous solution of equimolar amounts of calcium chloride and glycine was evaporated to dryness (see Materials and Methods). The resulting solid differed from the equimolar NaCl-Hgly mixture (see above) in that it was not a physical mixture of the starting components, but instead consisted of the newly formed coordination compound CaCl2(Hgly) · H2O. This compound has been described by us before (Yusenko et al. 2008). Its infrared spectrum clearly differed from those of α- and γ-glycine (Fig. 3, spectra A, B and D). Moreover, the X-ray powder diffraction pattern of CaCl2(Hgly) · H2O clearly differed from those of all three known polymorphs of glycine. The glycine diffraction patterns were calculated from single-crystal data (Brandenburg 2015; α-form: Langan et al. 2002; β-form: Ferrari et al. 2003; γ-form: Kvick et al. 1980). It is almost certain that the glycine molecules in CaCl2(Hgly) · H2O are directly bonded to Ca2+, just as in the closely related compound CaCl2(Hgly) · 3H2O (Yusenko et al. 2008).
Samples of CaCl2(Hgly) · H2O were kept at different temperatures for 7 days. The residue obtained was peach at 200 °C, light orange at 230 °C, brown at 250 °C, and black at 350 °C. At 200 °C, 94 % of the initial glycine had survived. The corresponding residue was mainly composed of intact CaCl2(Hgly) · H2O as revealed by its infrared spectrum which was virtually identical to that of the starting material (Fig. 3, spectra D and E). At 230 °C, the survival rate (85 %) was lower but still surprisingly high. At 250 and 350 °C, however, the glycine amount decreased dramatically and became too low for quantification. These results were very different from those obtained for neat glycine (Fox et al. 2015) and for glycine embedded in clay minerals (see above). Obviously, in CaCl2(Hgly) · H2O, the close association to a metal ion effectively protected the amino acid up to 230 °C. Interestingly, the thermomelanoid was formed only at 350 °C. At this temperature, volatiles such as N-heterocycles (mainly pyrroles), N-methylacetamide, and acetamide were released. They were identified by GC-MS (this work; Yusenko et al. 2008). A mass loss of 39 % accompanied the formation of the volatiles. At all temperatures, DKP was detected by HPLC, but no linear peptides were found.
There are some close similarities between Mg2+ and Ca2+, for example with respect to their preference for “hard” ligands (see for example: Pearson 1968; Parr and Pearson 1983) and the stability constants of their 1:1 complexes with neutral glycine (Casale et al. 1995). In fact, at least two well-characterized MgCl2-Hgly compounds are known (Yusenko et al. 2008, and references therein). Though we did not study the thermal behavior of these compounds, a protective effect comparable to that of Ca2+ may be expected for Mg2+.
Artificial Sea Salt-Glycine Mixture (AS-Hgly)
The AS-Hgly mixture contained NaCl, KCl, and glycine in the same ratio as the NaCl-KCl-Hgly mixture (see above), and, additionally, MgCl2 and CaCl2 (see Materials and Methods). The cation ratio corresponded to the average one in modern seawater (see for example: Killops and Killops 2005). On the anion side, only chloride was employed. The reasons why this composition was chosen as a model for Hadean–Early Archean sea salt have been discussed by Fox and Strasdeit (2013). The infrared spectrum and the X-ray powder diffractogram of the AS-Hgly mixture proved the presence of CaCl2(Hgly) · H2O. There were no indications of other glycine compounds or free glycine in any of its three modifications. Thus, CaCl2(Hgly) · H2O was the only or at least by far the major state of glycine in the AS-Hgly mixture.
As a consequence, glycine embedded in artificial sea salt showed similar thermal behavior as neat CaCl2(Hgly) · H2O. For instance, after heating AS-Hgly at 200 °C for 7 days, the sample was still white. Thus, the black thermomelanoid had not formed. Sublimation was also not observed. Besides glycine, DKP could be detected by HPLC in the aqueous solution of the residue. The survival rate of glycine was somewhat lower than in the corresponding experiment with neat CaCl2(Hgly) · H2O (84 % vs. 94 %), but the close association between the amino acid and Ca2+ provided substantial protection in the artificial sea salt matrix too.
Gypsum-Glycine and Magnesite-Glycine Mixtures
The main reason why we included gypsum (CaSO4 · 2H2O) and magnesite (MgCO3) in our study was because of their occurrence on Mars (Gendrin et al. 2005; Ehlmann et al. 2008). In the magnesite-Hgly mixture, glycine was present as the α-form. This was expected because the mixture had been prepared by dry mixing α-glycine and magnesite (see Materials and Methods). The relatively low concentration in the gypsum-glycine mixture made it more difficult to unequivocally determine the state of the amino acid. However, from the infrared spectrum it was reasonably certain that this mixture also contained α-glycine.
Both mixtures were thermally treated at 200 °C. After 7 days, the residues were dark grey (gypsum-Hgly) and light grey (magnesite-Hgly), respectively. We suspect that the grey color was caused by the thermomelanoid. However, this could not be proven because the low solubilities impeded the separation of the dark material from the matrices. In both experiments, glycine and DKP were detected in the residue and in the sublimate which had been formed. The residue of gypsum-Hgly contained only 10 % of the initial amount of glycine. The amount of DKP was not sufficient for quantification. Thus, the protection against decomposition and sublimation was weak. In the case of magnesite-Hgly, HPLC quantification was not possible because of interfering peaks from unidentified decomposition products. The simple presence of unreacted glycine, however, indicated a certain, albeit probably small, protective effect of magnesite.
Volcanic Rock and Martian Regolith Simulants
Volcanic Rock-Glycine Mixture
The volcanic rock used was a picrite, i.e., a basalt rich in olivine (see Materials and Methods). Picritic basalts bear some chemical resemblance to komatiites, especially in their high magnesium content (see for example: Gill 2010). Komatiites are igneous rocks that mainly formed in the Archean eon. They may have been relevant to the origin and early evolution of life (Nna-Mvondo and Martinez-Frias 2007). Basalts in general “are by far the most abundant rock type on Earth (and possibly in the inner planets)” (Best 2003). In fact, basalts, including picrobasalt, have also been identified on Mars (McSween et al. 2009; Taylor and McLennan 2009). Therefore, it seemed appropriate to use a picritic basalt as matrix material in our study.
A picrite-Hgly mixture, which contained 466 μmol of the amino acid, was heated at 200 °C for 2 days. HPLC analysis of the water extract of the residue revealed the presence of a small amount of DKP (3 μmol). Interestingly, glycine and other glycine peptides were absent. A sublimate composed of glycine and DKP was observed, but its amount was too low to account for the loss of glycine. Therefore, the residue was analyzed for the thermomelanoid. To this end, it was vigorously shaken with carbon tetrachloride, whose density (1.59 g cm−3 at 20 °C) is higher than that of the thermomelanoid (1.50 g cm−3 at 21 °C, Fox et al. 2015). In this process, the heavier mineral grains separated from black particles which floated on top of the liquid. The black particles were collected and dried. Their infrared spectrum was of rather poor quality but sufficed to identify the thermomelanoid. The overall results showed that virtually all glycine sublimed or was transformed into DKP and the thermomelanoid. Thus, picritic basalt has almost no protective effect.
Mixtures of Martian Regolith Simulants and Glycine
Three Martian regolith simulants were used as matrix materials for glycine, namely P-MRS, S-MRS, and JSC Mars-1A (see Materials and Methods, and Table 4). P-MRS is mainly composed of the clay minerals montmorillonite and chamosite (Böttger et al. 2012). A glycine-loaded P-MRS sample was heated at 200 °C for 2 days. The HPLC analysis of the residue showed the presence of glycine, DKP, and the linear peptides from diglycine to hexaglycine. This result is qualitatively identical to those obtained with the montmorillonites SAz-1 and SAz-1-Na (see above). Obviously, the intercalation of glycine into the clay minerals of P-MRS promoted polymerization and protected the amino acid from complete decomposition.
In contrast, in an S-MRS-Hgly sample, after heating under identical conditions, only glycine, DKP, and diglycine were found. Higher peptides were absent. S-MRS mainly contains igneous rocks and gypsum, but no clay minerals (Table 4). In our experiments with the igneous rock picrite and gypsum, the only peptide detectable was DKP (see above). The additional formation of diglycine in S-MRS was possibly caused by goethite and hematite which are known to promote the condensation of glycine to diglycine (Shanker et al. 2012). In the absence of clay minerals, however, no higher peptides formed in S-MRS. In both the experiment with P-MRS and the experiment with S-MRS, a sublimate composed of glycine and DKP was observed.
The Martian regolith simulant JSC Mars-1A had been collected on the Island of Hawaii (Allen et al. 1997). A blank experiment was conducted to exclude contaminants, particularly glycine and glycine peptides, that could have interfered with the HPLC analyses (see Materials and Methods). The main constituent of JSC Mars-1A is palagonite, a product that forms by hydration of volcanic glass at low temperature (Table 4). The phyllosilicate content is less than 1 %. A JSC Mars-1A-Hgly mixture was exposed to 200 °C for 2 days. In the residue, only DKP and unreacted glycine could be detected by HPLC. Sublimed DKP and glycine were also observed. While the glycine content of the starting mixture was 7.0 %, the overall mass loss in the experiment was about twice as large (15 %). However, it was known that the matrix contained water (Allen et al. 1997). Therefore, we heated a sample of neat JSC Mars-1A at 200 °C for 2 days. In this experiment, a water content of 11 % was found. Thus, the mass loss of the glycine-loaded material was mainly due to the release of water from the Martian regolith simulant. As with P-MRS-Hgly and S-MRS-Hgly, HPLC quantification was unfortunately prevented by insufficient peak resolution, which was partly caused by signals from unidentified products.
Summary and Conclusions
At temperatures around 200 °C, neat glycine forms an insoluble polymeric “thermomelanoid”, which is only slowly hydrolyzed in neutral aqueous medium and therefore can be regarded as a prebiotic storage form of the amino acid (Fox et al. 2015). Our present study shows that astrobiologically relevant minerals and mineral mixtures can alter the thermal behavior of glycine, sometimes drastically. Depending on the nature of the mineral matrix, different degrees of protection against thermal transformation at 200 °C were identified: (i) nearly complete protection, with more than 90 % of the amino acid being unaffected by the thermal treatment; (ii) lower but still substantial survival rates of glycine associated with the formation of considerable amounts of small glycine homopeptides, especially DKP; (iii) low glycine survival rates in the samples (~10 % or less) and minor formation of peptides (often exclusively DKP) accompanied by thermomelanoid formation and sublimation as the main processes.
In our study, the best protection was provided in CaCl2(Hgly) · H2O, which probably contains glycine directly bonded to Ca2+. This compound was also present in our artificial sea salt-glycine mixture. Consequently, the thermal survival rate of the amino acid in this mixture was similarly high. In the absence of calcium chloride, magnesium chloride will bind glycine and may exert a comparable protective effect (see above). Given that the Hadean–Early Archean ocean was probably salty (Knauth 1998), it is reasonable to assume that Ca2+ and/or Mg2+ were among the dissolved ions. If glycine was also present in the seawater, we can conclude that sea salt crusts, for example at hot volcanic coasts, prevented the thermal decomposition of the amino acid up to temperatures slightly above 200 °C. Compound formation between glycine and the metal salt is crucial to a highly effective protection. When only physical mixtures exist (as with NaCl, NaCl-KCl, gypsum, and magnesite), the survival rate of the amino acid is considerably lower. Coordination to Ca2+ and Mg2+ presents no problem to the prebiotic availability of glycine because the stability of the M(Hgly)2+ complexes in solution is low (Casale et al. 1995).
Among the five clay minerals studied, the ability to protect glycine varied strongly. At 200 °C, by far the lowest survival rate was observed with the kaolinite KGa-1. In contrast to the other clay minerals, which were smectites, KGa-1 very probably did not intercalate glycine. Thus, intercalation is obviously a stabilizing factor. Substantial differences in the survival rate occurred even between closely related minerals, for example the Ca-montmorillonites SAz-1 and STx-1. Therefore, at present, quantitative predictions for clays seem impossible. Another interesting result was the observation that all clay minerals that have been studied favored the thermal formation of glycine peptides, with DKP being the dominating product. In the case of the montmorillonite SAz-1, all linear homopeptides up to hexaglycine (Gly6) were identified. From the viewpoint of prebiotic chemical evolution, SAz-1 appears to be exceptionally effective: after exposure to 200 °C for 2 days, ~95 % of the glycine had either survived or been transformed into peptides. Even at 250 °C, other processes such as thermomelanoid formation and sublimation were largly suppressed. Especially at this higher temperature, the cyclic dipeptide DKP was formed at high yield (~68 %). It should be noted that DKP is not necessarily a dead end in prebiotic syntheses (Georgelin et al. 2013; Fuchida et al. 2014).
Glycine in picritic basalt—an igneous rock—showed the same qualitative thermal behavior as the neat form, i.e., formation of the thermomelanoid and small amounts of DKP accompanied by sublimation. Thus, in this case, the effect of the matrix was neglectable. Of the three Martian regolith simulants that we used in our study, two contained no or very little phyllosilicates, but were mainly composed of igneous rocks and gypsum (S-MRS) and the basalt alteration product palagonite (JSC Mars-1A), respectively. Consistent with these compositions, heating of glycine-loaded S-MRS and JSC Mars-1A at 200 °C for 2 days produced no higher peptides. In contrast, the Martian regolith simulant P-MRS, which is mainly composed of phyllosilicates, promoted the formation of peptides up to hexaglycine.
In conclusion, we have demonstrated that some prebiotically relevant minerals had profound effects on the survival of glycine under conditions of dry heating. Some evaporites and phyllosilicates were especially effective in protecting glycine. As both types of minerals are also found on Mars (see Introduction), these results are not only significant for the early Earth. Certain phyllosilicates—in neat form or as constituents of a Martian regolith simulant—promoted the formation of small peptides at temperatures that must have occurred near volcanic eruptions and meteorite impacts on the young Earth and Mars. We note, however, that both the duration of heat production and the temperature in the vicinity of these local heat sources could have covered a wide range.
Glycine is among the prebiotically most important amino acids (Zaia et al. 2008). In order to assess its possible relevance to the origin of life, it is necessary not only to estimate the sources of the amino acid, but also its sinks. Thermal decomposition was clearly a prebiotic sink. Our findings, however, indicate that the protective effect of certain minerals made glycine less vulnerable to higher temperatures, which would have resulted in higher concentrations of the amino acid. This result strengthens the case for glycine being one of the molecules involved in life’s origin, at least if the latter was heterotrophic in nature.
References
Allen CC, Morris RV, Lindstrom DJ, Lindstrom MM, Lockwood JP (1997) JSC Mars-1: a Martian regolith simulant. Presented at the 28th lunar and planetary science conference, Houston, Texas, USA, paper 1797.pdf
Ambujam K, Selvakumar S, Anand DP, Mohamed G, Sagayaraj P (2006) Crystal growth, optical, mechanical and electrical properties of organic NLO material γ-glycine. Cryst Res Technol 41:671–677
Arndt NT, Nisbet EG (2012) Processes on the young Earth and the habitats of early life. Annu Rev Earth Planet Sci 40:521–549
Bandfield JL, Glotch TD, Christensen PR (2003) Spectroscopic identification of carbonate minerals in the Martian dust. Science 301:1085–1087
Basiuk VA, Navarro-González R (1996) Possible role of volcanic ash-gas clouds in the Earth’s prebiotic chemistry. Orig Life Evol Biosph 26:173–194
Benincasa E, Brigatti MF, Lugli C, Medici L, Poppi L (2000) Interaction between glycine and Na-, Ca- and Cu-rich smectites. Clay Miner 35:635–641
Bernal JD (1949) The physical basis of life. Proc Phys Soc B 62:597–618
Bertrand M, van der Gaast S, Vilas F, Hörz F, Haynes G, Chabin A, Brack A, Westall F (2009) The fate of amino acids during simulated meteoritic impact. Astrobiology 9:943–951
Best MG (2003) Igneous and metamorphic petrology, 2nd edn. Blackwell, Malden, p 37
Bhushan R, Brückner H (2004) Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247
Bibring J-P, Langevin Y, Gendrin A, Gondet B, Poulet F, Berthé M, Soufflot A, Arvidson R, Mangold N, Mustard J, Drossart P, the OMEGA team (2005) Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 307:1576–1581
Bibring J-P, Langevin Y, Mustard JF, Poulet F, Arvidson R, Gendrin A, Gondet B, Mangold N, Pinet P, Forget F, the OMEGA team (2006) Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 312:400–404
Biron J-P, Pascal R (2004) Amino acid N-carboxyanhydrides: activated peptide monomers behaving as phosphate-activating agents in aqueous solution. J Am Chem Soc 126:9198–9199
Biron J-P, Parkes AL, Pascal R, Sutherland JD (2005) Expeditious, potentially primordial, aminoacylation of nucleotides. Angew Chem Int Ed 44:6731–6734
Bishop JL, Noe Dobrea EZ, McKeown NK, Parente M, Ehlmann BL, Michalski JR, Milliken RE, Poulet F, Swayze GA, Mustard JF, Murchie SL, Bibring J-P (2008) Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 321:830–833
Borden D, Giese RF (2001) Baseline studies of the Clay Minerals Society source clays: cation exchange capacity measurements by the ammonia-electrode method. Clays Clay Miner 49:444–445
Böttger U, de Vera J-P, Fritz J, Weber I, Hübers H-W, Schulze-Makuch D (2012) Optimizing the detection of carotene in cyanobacteria in a Martian regolith analogue with a Raman spectrometer for the ExoMars mission. Planet Space Sci 60:356–362
Brack A (2013) Clay minerals and the origin of life. In: Bergaya F, Lagaly G (eds) Handbook of clay science, part A: fundamentals, 2nd edn. Elsevier, Amsterdam, pp 507–521
Brandenburg K (2015) Diamond – Crystal and molecular structure visualization, version 4.0.5, Crystal Impact, Bonn, Germany
Buick R, Dunlop JSR (1990) Evaporitic sediments of Early Archaean age from the Warrawoona Group, North Pole, Western Australia. Sedimentology 37:247–277
Buick R, Thornett JR, McNaughton NJ, Smith JB, Barley ME, Savage M (1995) Record of emergent continental crust ~3.5 billion years ago in the Pilbara craton of Australia. Nature 375:574–577
Bujdák J, Rode BM (1996) The effect of smectite composition on the catalysis of peptide bond formation. J Mol Evol 43:326–333
Bujdák J, Rode BM (1999) Silica, alumina and clay catalyzed peptide bond formation: enhanced efficiency of alumina catalyst. Orig Life Evol Biosph 29:451–461
Casale A, De Robertis A, De Stefano C, Gianguzza A, Patanè G, Rigano C, Sammartano S (1995) Thermodynamic parameters for the formation of glycine complexes with magnesium(II), calcium(II), lead(II), manganese(II), cobalt(II), nickel(II), zinc(II) and cadmium(II) at different temperatures and ionic strengths, with particular reference to natural fluid conditions. Thermochim Acta 255:109–141
Chevrier V, Mathé PE (2007) Mineralogy and evolution of the surface of Mars: a review. Planet Space Sci 55:289–314
Cleaves HJ, Aubrey AD, Bada JL (2009) An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems. Orig Life Evol Biosph 39:109–126
Cleaves HJ II, Scott AM, Hill FC, Leszczynski J, Sahai N, Hazen R (2012) Mineral–organic interfacial processes: potential roles in the origins of life. Chem Soc Rev 41:5502–5525
Cloutis EA, Grasby SE, Last WM, Leveille R, Osinski GR, Sherriff BL (2010) Spectral reflectance properties of carbonates from terrestrial analogue environments: implications for Mars. Planet Space Sci 58:522–537
Córdoba-Jabonero C, Lara LM, Mancho AM, Márquez A, Rodrigo R (2003) Solar ultraviolet transfer in the Martian atmosphere: biological and geological implications. Planet Space Sci 51:399–410
Dalai P (2013) Thermal behavior of amino acids in inorganic matrices: relevance for chemical evolution. Doctoral thesis. University of Hohenheim, 139 pp
Dalai P, Strasdeit H (2009) The Influence of a clay mineral on the behavior of glycine at 200 degrees Celsius. Orig Life Evol Biosph 39:47–48
Deamer DW (2004) Prebiotic amphiphilic compounds: self-assembly and properties of early membrane structures. In: Seckbach J (ed) Origins: genesis, evolution and diversity of life. Kluwer, Dordrecht, pp 75–89
Eberl DD (1984) Clay mineral formation and transformation in rocks and soils. Phil Trans R Soc Lond A 311:241–257
Ehlmann BL, Mustard JF, Murchie SL, Poulet F, Bishop JL, Brown AJ, Calvin WM, Clark RN, Des Marais DJ, Milliken RE, Roach LH, Roush TL, Swayze GA, Wray JJ (2008) Orbital identification of carbonate-bearing rocks on Mars. Science 322:1828–1832
Ferrari ES, Davey RJ, Cross WI, Gillon AL, Towler CS (2003) Crystallization in polymorphic systems: the solution-mediated transformation of β to α glycine. Cryst Growth Des 3:53–60
Fishbaugh KE, Poulet F, Chevrier V, Langevin Y, Bibring J-P (2007) On the origin of gypsum in the Mars north polar region. J Geophys Res 112:1–17
Fox S, Strasdeit H (2013) Possible prebiotic origin on volcanic islands of oligopyrrole-type photopigments and electron transfer cofactors. Astrobiology 13:578–595
Fox S, Dalai P, Lambert J-F, Strasdeit H (2015) Hypercondensation of an amino acid: synthesis and characterization of a black glycine polymer. Chem Eur J 21:8897–8904
Fuchida S, Masuda H, Shinoda K (2014) Peptide formation mechanism on montmorillonite under thermal conditions. Orig Life Evol Biosph 44:13–28
Fujio H, Noma Y, Amano T (1959) Analytical aspects of the precipitin reaction using some artificial antigens. Biken J 2:35–49, CAplus accession number: 1960:131194
Futamura Y, Fujioka K, Yamamoto K (2008) Hydrothermal treatment of glycine and adiabatic expansion cooling: implications for prebiotic synthesis of biopolymers. J Mater Sci 43:2442–2446
Galán E, Ferrell RE (2013) Genesis of clay minerals. In: Bergaya F, Lagaly G (eds) Handbook of clay science, part A: fundamentals, 2nd edn. Elsevier, Amsterdam, pp 83–126
Gendrin A, Mangold N, Bibring J-P, Langevin Y, Gondet B, Poulet F, Bonello G, Quantin C, Mustard J, Arvidson R, LeMouélic S (2005) Sulfates in Martian layered terrains: the OMEGA/Mars Express view. Science 307:1587–1591
Georgelin T, Jaber M, Bazzi H, Lambert JF (2013) Formation of activated biomolecules by condensation on mineral surfaces – A comparison of peptide bond formation and phosphate condensation. Orig Life Evol Biosph 43:429–443
Gill R (2010) Igneous rocks and processes: a practical guide. Wiley-Blackwell, Chichester, pp 131–160
Glotch TD, Bandfield JL, Tornabene LL, Jensen HB, Seelos FP (2010) Distribution and formation of chlorides and phyllosilicates in Terra Sirenum, Mars. Geophys Res Lett 37:L16202
Hazen RM (2006) Mineral surfaces and the prebiotic selection and organization of biomolecules. Am Mineral 91:1715–1729
Hazen RM (2013) Paleomineralogy of the Hadean eon: a preliminary species list. Am J Sci 313:807–843
Hedges JI, Hare PE (1987) Amino acid adsorption by clay minerals in distilled water. Geochim Cosmochim Acta 51:255–259
Hill RD (1992) An efficient lightning energy source on the early Earth. Orig Life Evol Biosph 22:277–285
Huber C, Wächtershauser G (1998) Peptides by activation of amino acids with CO on (Ni, Fe)S surfaces: implications for the origin of life. Science 281:670–672
Imai E-i, Honda H, Hatori K, Brack A, Matsuno K (1999a) Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 283:831–833
Imai E-i, Honda H, Hatori K, Matsuno K (1999b) Autocatalytic synthesis of oligoglycine in a simulated submarine hydrothermal system. Orig Life Evol Biosph 29:249–259
Johnson AP, Cleaves HJ, Dworkin JP, Glavin DP, Lazcano A, Bada JL (2008) The Miller volcanic spark discharge experiment. Science 322:404
Jover J, Bosque R, Sales J (2008) A comparison of the binding affinity of the common amino acids with different metal cations. Dalton Trans 6441–6453. doi:10.1039/b805860a
Keeling JL, Raven MD, Gates WP (2000) Geology and characterization of two hydrothermal nontronites from weathered metamorphic rocks at the Uley graphite mine, South Australia. Clay Clay Miner 48:537–548
Killops S, Killops V (2005) Introduction to organic geochemistry, 2nd edn. Blackwell, Malden, p 72
Kitadai N, Yokoyama T, Nakashima S (2011) Hydration–dehydration interactions between glycine and anhydrous salts: implications for a chemical evolution of life. Geochim Cosmochim Acta 75:6285–6299
Knauth LP (1998) Salinity history of the Earth’s early ocean. Nature 395:554–555
Krumm S (1994) Atterberg: a program for calculation of settling times, grain diameters, falling heights. For a newer version, see: http://www.gzn.uni-erlangen.de/krustendynamik/mitarbeiter/akademische-mitarbeiter/krumm/software. Accessed 16 Aug 2016
Kuzmin RO, Mironenko MV, Evdokimova NA (2009) Spectral and thermodynamic constraints on the existence of gypsum at the Juventae Chasma on Mars. Planet Space Sci 57:975–981
Kvick Å, Canning WM, Koetzle TF, Williams GJB (1980) An experimental study of the influence of temperature on a hydrogen-bonded system: the crystal structure of γ-glycine at 83 K and 298 K by neutron diffraction. Acta Crystallogr B 36:115–120
Lagaly G, Ogawa M, Dékány I (2013) Clay mineral–organic interactions. In: Bergaya F, Lagaly G (eds) Handbook of clay science, part A: fundamentals, 2nd edn. Elsevier, Amsterdam, pp 435–505
Lahav N, White DH (1980) A possible role of fluctuating clay-water systems in the production of ordered prebiotic oligomers. J Mol Evol 16:11–21
Lahav N, White DH, Chang S (1978) Peptide formation in the prebiotic era: thermal condensation of glycine in fluctuating clay environments. Science 201:67–69
Lambert JF (2008) Adsorption and polymerization of amino acids on mineral surfaces: a review. Orig Life Evol Biosph 38:211–242
Langan P, Mason SA, Myles D, Schoenborn BP (2002) Structural characterization of crystals of α-glycine during anomalous electrical behaviour. Acta Crystallogr B 58:728–733
Lellouch E, Encrenaz T, de Graauw T, Erard S, Morris P, Crovisier J, Feuchtgruber H, Girard T, Burgdorf M (2000) The 2.4–45 μm spectrum of Mars observed with the Infrared Space Observatory. Planet Space Sci 48:1393–1405
Leman L, Orgel L, Ghadiri MR (2004) Carbonyl sulfide–mediated prebiotic formation of peptides. Science 306:283–286
Leman LJ, Orgel LE, Ghadiri MR (2006) Amino acid dependent formation of phosphate anhydrides in water mediated by carbonyl sulfide. J Am Chem Soc 128:20–21
Mangold N, Poulet F, Mustard JF, Bibring J-P, Gondet B, Langevin Y, Ansan V, Masson P, Fassett C, Head JW III, Hoffmann H, Neukum G (2007) Mineralogy of the Nili Fossae region with OMEGA/Mars Express data: 2. Aqueous alteration of the crust. J Geophys Res 112:E08S04
Martins Z, Sephton MA (2009) Extraterrestrial amino acids. In: Hughes AB (ed) Amino acids, peptides and proteins in organic chemistry, vol 1. Wiley-VCH, Weinheim, pp 3–42
Martra G, Deiana C, Sakhno Y, Barberis I, Fabbiani M, Pazzi M, Vincenti M (2014) The formation and self-assembly of long prebiotic oligomers produced by the condensation of unactivated amino acids on oxide surfaces. Angew Chem Int Ed 53:4671–4674
McSween HY Jr (1994) What we have learned about Mars from SNC meteorites. Meteoritics 29:757–779
McSween HY Jr, Taylor GJ, Wyatt MB (2009) Elemental composition of the Martian crust. Science 324:736–739
Meng M, Xia L-Y, Guo L-H (2007) Adsorption and thermal condensation of glycine on kaolinite. Acta Phys-Chim Sin 23:32–36
Mermut AR, Cano AF (2001) Baseline studies of the Clay Minerals Society source clays: chemical analyses of major elements. Clays Clay Miner 49:381–386
Miller SL (1953) A production of amino acids under possible primitive Earth conditions. Science 117:528–529
Miller SL (1955) Production of some organic compounds under possible primitive Earth conditions. J Am Chem Soc 77:2351–2361
Morris RV, Ruff SW, Gellert R, Ming DW, Arvidson RE, Clark BC, Golden DC, Siebach K, Klingelhöfer G, Schröder C, Fleischer I, Yen AS, Squyres SW (2010) Identification of carbonate-rich outcrops on Mars by the Spirit Rover. Science 329:421–424
Mustard JF, Murchie SL, Pelkey SM, Ehlmann BL, Milliken RE, Grant JA, Bibring J-P, Poulet F, Bishop J, Noe Dobrea E, Roach L, Seelos F, Arvidson RE, Wiseman S, Green R, Hash C, Humm D, Malaret E, McGovern JA, Seelos K, Clancy T, Clark R, Des Marais D, Izenberg N, Knudson A, Langevin Y, Martin T, McGuire P, Morris R, Robinson M, Roush T, Smith M, Swayze G, Taylor H, Titus T, Wolff M (2008) Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 454:305–309
Nair NN, Schreiner E, Marx D (2008) Peptide synthesis in aqueous environments: the role of extreme conditions on amino acid activation. J Am Chem Soc 130:14148–14160
Navarro-González R, Segura A (2004) The possible role of volcanic lightning in chemical evolution. In: Seckbach J (ed) Origins: genesis, evolution and diversity of life. Kluwer, Dordrecht, pp 137–152
Nimmo F, Tanaka K (2005) Early crustal evolution of Mars. Annu Rev Earth Planet Sci 33:133–161
Nisbet EG, Fowler CMR (2004) The early history of life. In: Schlesinger WH (ed) Biogeochemistry; Vol. 8 of Holland HD, Turekian KK (eds) Treatise on geochemistry. Elsevier Pergamon, Oxford, pp 1–39
Nna-Mvondo D, Martinez-Frias J (2007) Review komatiites: from Earth’s geological settings to planetary and astrobiological contexts. Earth Moon Planets 100:157–179
Noe Dobrea EZ, Poulet F, Malin MC (2008) Correlations between hematite and sulfates in the chaotic terrain east of Valles Marineris. Icarus 193:516–534
Odom IE (1984) Smectite clay minerals: properties and uses. Phil Trans R Soc Lond A 311:391–409
Ohara S, Kakegawa T, Nakazawa H (2007) Pressure effects on the abiotic polymerization of glycine. Orig Life Evol Biosph 37:215–223
Olsen JV, de Godoy LMF, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 4:2010–2021
Osterloo MM, Hamilton VE, Bandfield JL, Glotch TD, Baldridge AM, Christensen PR, Tornabene LL, Anderson FS (2008) Chloride–bearing materials in the southern highlands of Mars. Science 319:1651–1654
Otake T, Taniguchi T, Furukawa Y, Kawamura F, Nakazawa H, Kakegawa T (2011) Stability of amino acids and their oligomerization under high-pressure conditions: implications for prebiotic chemistry. Astrobiology 11:799–813
Palomba E, Zinzi A, Cloutis EA, D’Amore M, Grassi D, Maturilli A (2009) Evidence for Mg-rich carbonates on Mars from a 3.9 μm absorption feature. Icarus 203:58–65
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Pearson RG (1968) Hard and soft acids and bases, HSAB, part I: fundamental principles. J Chem Educ 45:581–587
Pizzarello S, Cooper GW, Flynn GJ (2006) The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta DS, McSween HY Jr (eds) Meteorites and the early solar system II. The University of Arizona Press, Tucson, pp 625–651
Poch O, Jaber M, Stalport F, Nowak S, Georgelin T, Lambert J-F, Szopa C, Coll P (2015) Effect of nontronite smectite clay on the chemical evolution of several organic molecules under simulated Martian surface ultraviolet radiation conditions. Astrobiology 15:221–237
Ponnamperuma C, Shimoyama A, Friebele E (1982) Clay and the origin of life. Orig Life Evol Biosph 12:9–40
Poulet F, Bibring J-P, Mustard JF, Gendrin A, Mangold N, Langevin Y, Arvidson RE, Gondet B, Gomez C, the Omega Team (2005) Phyllosilicates on Mars and implications for early Martian climate. Nature 438:623–627
Poulet F, Mangold N, Loizeau D, Bibring J-P, Langevin Y, Michalski J, Gondet B (2008) Abundance of minerals in the phyllosilicate-rich units on Mars. Astron Astrophys 487:L41–L44
Remko M, Rode BM (2001) Catalyzed peptide bond formation in the gas phase. Phys Chem Chem Phys 3:4667–4673
Remko M, Rode BM (2004) Catalyzed peptide bond formation in the gas phase. Role of bivalent cations and water in formation of 2-aminoacetamide from ammonia and glycine and in dimerization of glycine. Struct Chem 15:223–232
Rieder R, Economou T, Wänke H, Turkevich A, Crisp J, Brückner J, Dreibus G, McSween HY Jr (1997) The chemical composition of Martian soil and rocks returned by the mobile alpha proton X-ray spectrometer: preliminary results from the X-ray mode. Science 278:1771–1774
Righi D, Meunier A (1995) Origin of clays by rock weathering and soil formation. In: Velde B (ed) Origin and mineralogy of clays. Springer, Berlin, pp 43–161
Rode BM, Schwendinger MG (1990) Copper-catalyzed amino acid condensation in water – A simple possible way of prebiotic peptide formation. Orig Life Evol Biosph 20:401–410
Sato M (1999) Preparation of kaolinite-amino acid intercalates derived from hydrated kaolinite. Clay Clay Miner 47:793–802
Schlesinger G, Miller SL (1983) Prebiotic synthesis in atmospheres containing CH4, CO, and CO2 I. Amino acids. J Mol Evol 19:376–382
Schwendinger MG, Rode BM (1989) Possible role of copper and sodium chloride in prebiotic evolution of peptides. Anal Sci 5:411–414
Shanker U, Bhushan B, Bhattacharjee G, Kamaluddin (2012) Oligomerization of glycine and alanine catalyzed by iron oxides: implications for prebiotic chemistry. Orig Life Evol Biosph 42:31–45
Shock EL, Schulte MD (1990) Summary and implications of reported amino acid concentrations in the Murchison meteorite. Geochim Cosmochim Acta 54:3159–3173
Sleep NH (2010) The Hadean-Archaean environment. Cold Spring Harb Perspect Biol 2:a002527
Srinivasan K, Arumugam J (2007) Growth of non-linear optical γ-glycine single crystals and their characterization. Opt Mater 30:40–43
Strasdeit H (2010) Chemical evolution and early Earth’s and Mars’ environmental conditions. Palaeodiversity Suppl 3:107–116
Sugahara H, Mimura K (2014a) Shock-induced pyrolysis of amino acids at ultra high pressures ranged from 3.2 to 35.3 GPa. J Anal Appl Pyrolysis 108:170–175
Sugahara H, Mimura K (2014b) Glycine oligomerization up to triglycine by shock experiments simulating comet impacts. Geochem J 48:51–62
Taylor SR, McLennan SM (2009) Planetary crusts: their composition, origin and evolution. Cambridge University Press, Cambridge, pp 141–180, 233–274
The Clay Minerals Society (2016) Source clay physical/chemical data. http://www.clays.org/Sourceclays.html. Accessed 16 Aug 2016
Tsukahara H, Imai E-i, Honda H, Hatori K, Matsuno K (2002) Prebiotic oligomerization on or inside lipid vesicles in hydrothermal environments. Orig Life Evol Biosph 32:13–21
Van Kranendonk MJ, Webb GE, Kamber BS (2003) Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean. Geobiology 1:91–108
Van Kranendonk MJ, Philippot P, Lepot K, Bodorkos S, Pirajno F (2008) Geological setting of Earth’s oldest fossils in the ca. 3.5 Ga Dresser formation, Pilbara Craton, Western Australia. Precambrian Res 167:93–124
Wang A, Freeman JJ, Jolliff BL, Chou I-M (2006) Sulfates on Mars: a systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochim Cosmochim Acta 70:6118–6135
Westall F, de Ronde CEJ, Southam G, Grassineau N, Colas M, Cockell C, Lammer H (2006) Implications of a 3.472–3.333 Gyr-old subaerial microbial mat from the Barberton greenstone belt, South Africa for the UV environmental conditions on the early Earth. Phil Trans R Soc B 361:1857–1875
Wray JJ, Squyres SW, Roach LH, Bishop JL, Mustard JF, Noe Dobrea EZ (2010) Identification of the Ca-sulfate bassanite in Mawrth Vallis, Mars. Icarus 209:416–421
Xiao L, Huang J, Christensen PR, Greeley R, Williams DA, Zhao J, He Q (2012) Ancient volcanism and its implication for thermal evolution of Mars. Earth Planet Sci Lett 323–324:9–18
Yusenko K, Fox S, Guni P, Strasdeit H (2008) Model studies on the formation and reactions of solid glycine complexes at the coasts of a primordial salty ocean. Z Anorg Allg Chem 634:2347–2354
Zaia DAM, Zaia CTBV, De Santana H (2008) Which amino acids should be used in prebiotic chemistry studies? Orig Life Evol Biosph 38:469–488
Zheng W, Zhou J, Zhang Z, Chen L, Zhang Z, Li Y, Ma N, Du P (2014) Formation of intercalation compound of kaolinite–glycine via displacing guest water by glycine. J Colloid Interface Sci 432:278–284
Acknowledgments
We thank Claudia Görgen, Sonja Ringer, Melanie Wagner, and the late Petra Guni for technical assistance. The authors are grateful to Thomas Staudacher (Observatoire volcanologique du Piton de la Fournaise) for providing the picritic basalt and to Dr. Manfred Martin (Regierungspräsidium Freiburg; Landesamt für Geologie, Rohstoffe und Bergbau) for its elemental analysis. We also thank Dr. Jean-Pierre Paul de Vera (DLR Institute of Planetary Research, Berlin) for providing samples of P-MRS and S-MRS. HLP is supported by an LGFG doctoral fellowship from the State of Baden-Württemberg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalai, P., Pleyer, H.L., Strasdeit, H. et al. The Influence of Mineral Matrices on the Thermal Behavior of Glycine. Orig Life Evol Biosph 47, 427–452 (2017). https://doi.org/10.1007/s11084-016-9523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-016-9523-0