Abstract
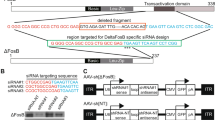
Heightened dopaminergic activity has been shown to be implicated in some major neuropsychiatric disorders such as schizophrenia. Use of dopaminergic antagonists was limited by some serious side effects related to unspecific blocking of dopamine receptors. Thus a target specific dopamine receptor gene silencing method such as using small interfering RNA (siRNA) might be useful. In this study recombinant plasmids expressing siRNA against dopamine receptors (D1-D5DRs) were produced, and their efficiency in knocking down of receptors in were assessed in rat neuroblastoma cell line (B65), using Real-time PCR method. Furthermore, D2DR siRNA expressing plasmid was injected into the rat nucleus accumbens bilaterally to investigate whether it can prevent the hyperactivity induced by apomorphine. Locomotion was measured in 10 min intervals, 50 min before and 60 min after apomorphine injection (0.5 mg/kg, S.C). Our results indicated that the mRNA level of dopamine receptors were reduced between 25 and 75% in B65 cells treated with the plasmids in vitro. In behavioral tests, locomotion was lower at least in the second 10 min after apomorphine injection in rats treated with plasmid expressing D2DR siRNA compare to control group [F (4,24) = 2.77, (P < 0.05)]. The spontaneous activity of treated rats was normal. In conclusion, dopamine receptors can be downregulated by use of siRNA expressing plasmids in nucleus accumbens. Although our work may have some possible clinical applications; the potentially therapeutic application of siRNA in knocking down of dopamine receptors needs further studies.




Similar content being viewed by others
References
Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10:376–384
Floresco SB, Tse MT, Ghods-Sharifi S (2008) Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33:1966–1979
Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30:220–227
Rosenkranz JA, Grace AA (1999) Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 19:11027–11039
Arnsten AF (2009) Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 23(Suppl 1):33–41
Bonci A, Bernardi G, Grillner P, Mercuri NB (2003) The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci 24:172–177
Greene JG (2006) Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J Physiol 575:411–416
Kebabian JW, Petzold GL, Greengard P (1972) Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc Natl Acad Sci USA 69:2145–2149
Seeman P, Chau-Wong M, Tedesco J, Wong K (1975) Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci USA 72:4376–4380
Werkman TR, Glennon JC, Wadman WJ, McCreary AC (2006) Dopamine receptor pharmacology: interactions with serotonin receptors and significance for the aetiology and treatment of schizophrenia. CNS Neurol Disord Drug Targets 5:3–23
Strange PG (1993) Dopamine receptors: structure and function. Prog Brain Res 99:167–179
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132
Birkmayer W, Hornykiewicz O (1961) The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr 73:787–788
Carlsson A, Lindqvist M (1963) Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 20:140–144
Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30:188–193
Sah DW (2006) Therapeutic potential of RNA interference for neurological disorders. Life Sci 79:1773–1780
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA et al (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223
Remington G, Kapur S (2010) Antipsychotic dosing: how much but also how often? Schizophr Bull 36:900–903
Schultz SH, North SW, Shields CG (2007) Schizophrenia: a review. Am Fam Physician 75:1821–1829
Aagaard L, Rossi JJ (2007) RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 59:75–86
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Kim DH, Rossi JJ (2007) Strategies for silencing human disease using RNA interference. Nat Rev Genet 8:173–184
Takahashi Y, Nishikawa M, Takakura Y (2009) Nonviral vector-mediated RNA interference: its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv Drug Deliv Rev 61:760–766
Thakker DR, Hoyer D, Cryan JF (2006) Interfering with the brain: use of RNA interference for understanding the pathophysiology of psychiatric and neurological disorders. Pharmacol Ther 109:413–438
Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV (2002) Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci 3:18
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Wadhwa R, Kaul SC, Miyagishi M, Taira K (2004) Know-how of RNA interference and its applications in research and therapy. Mutat Res 567:71–84
Backman C, Zhang Y, Hoffer BJ, Tomac AC (2003) Short interfering RNAs (siRNAs) for reducing dopaminergic phenotypic markers. J Neurosci Methods 131:51–56
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R et al (2000) Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97:8104–8109
Barr AM, Powell SB, Markou A, Geyer MA (2006) Iloperidone reduces sensorimotor gating deficits in pharmacological models, but not a developmental model, of disrupted prepulse inhibition in rats. Neuropharmacology 51:457–465
Breysse N, Risterucci C, Amalric M (2002) D1 and D2 dopamine receptors contribute to the locomotor response induced by Group II mGluRs activation in the rat nucleus accumbens. Pharmacol Biochem Behav 73:347–357
Acknowledgments
This research has been supported by the Iran National Science Foundation (INSF), grant no. 83088.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noori-Daloii, MR., Mojarrad, M., Rashidi-nezhad, A. et al. Use of siRNA in knocking down of dopamine receptors, a possible therapeutic option in neuropsychiatric disorders. Mol Biol Rep 39, 2003–2010 (2012). https://doi.org/10.1007/s11033-011-0947-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0947-3