Abstract
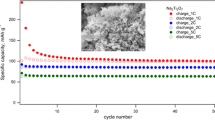
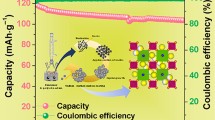
The NaVO3 sample has been successfully fabricated via a simple sol-gel method adding Na2CO3 and V2O5 powders into distilled water with citric acid to act as a chelating reagent. X-ray diffraction with Rietveld refinement result shows that single-phase NaVO3 can be obtained by our method. The differences caused by different sintering temperature exist in X-ray diffraction and scanning electron microscope (SEM), showing that sintering temperature has an important influence on crystal growth and grain size stabilization. When evaluated as an anode material for lithium-ion batteries, the nanosize NaVO3 electrode displays a discharge and recharge capacity of 623.8 and 355.6 mAh g−1 in the first cycle, while a reversible discharge-charge capacity of ∼250 mAh g−1 can be retained after 30 cycles. For comparison, the electrochemical properties of microsize NaVO3 prepared at a higher temperature are also displayed. Furthermore, the structure change of NaVO3 and its Li storage mechanism upon lithiation and delithiation process are studied by ex situ XRD and TEM in below.

ᅟ








Similar content being viewed by others
References
Goodenough JB, Park KS (2013) The Li-Ion Rechargeable Battery: a Perspective. J Am Chem Soc 135:1167–1176
Su L, Jing Y, Zhou Z (2011) Li ion battery materials with core-shell nanostructures. Nanoscale 3:3967–3983
Aric AS, Bruce P, Scrosati B, Tarascon JM, Schalkwijk WV (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377
Simon S, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Noorden RV (2014) The rechargeable revolution: a better battery. Nat Mater 507:26–28
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407:496–499
Laruelle S, Grugeon S, Poizot P, Dolle M, Dupont L, Tarascon JM (2002) On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J Electrochem Soc 149:A627–A634
Li WY, Xu LN, Chen J (2005) Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv Funct Mater 15:851–857
Taberna PL, Mitra S, Poizot P, Simon P, Tarascon JM (2006) High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nature Mater 5:567–573
Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ (2008) Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv Funct Mater 18:3941–3946
Bruce PG, Scrosati B, Tarascon JM (2008) Nanomaterials for rechargeable lithium batteries. Angew Chem Int Ed 47:2930–2946
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4301
Whittingham MS (1976) Electrical energy storage and intercalation chemistry. Science 192:1126–1127
Mishra KM, Lal AK, Haque FZ (2004) Ionic and electronic conductivity in some alkali vanadates. Solid State Ionics 167:137–146
Song JH, Park HJ, Kim KJ, Jo YN, Kim JS, Jeong YU, Kim YJ (2010) Electrochemical characteristics of lithium vanadate, Li1 + x VO2, new anode materials for lithium ion batteries. J Power Sources 195:6157–6161
West K, Zachau-Christiansen B, Jacobsen T, Atlung S (1985) V6O13 As cathode material for lithium cells. J Power Sources 14:235–245
Xiao L, Zhao Y, Yin J, Zhang L (2009) Clewlike ZnV2O4 Hollow Spheres: Nonaqueous Sol-Gel Synthesis, Formation Mechanism, and Lithium Storage Properties. Chem Eur J 15:9442–9450
Denis S, Baudrin E, Touboul M, Tarascon JM (1997) Synthesis and Electrochemical Properties of Amorphous Vanadates of General Formula RVO4 (R = In, Cr, Fe, Al, Y) vs. Li. J Electrochem Soc 144:4099–4109
Pistoia G, Pasquali M, Tocci M, Moshtev RV, Manev V (1985) Li/Li1 + x V3O8 Secondary Batteries III. Further Characterization of the Mechanism of Li+ Insertion and of the Cycling Behavior. J Electrochem Soc 32:281–284
Pralong V, Gopal V, Caignaert V, Duffort V, Raveau B (2012) Lithium-Rich Rock-Salt-Type Vanadate as Energy Storage Cathode: Li2–x VO3. Chem Mater 24:12–14
Qiao Y, Tu J, Wang X, Zhang J, Yu Y, Gu C (2011) Self-Assembled Synthesis of Hierarchical Waferlike Porous Li-V-O Composites as Cathode Materials for Lithium Ion Batteries. J Phys Chem C 115:25508–25518
Li H, Liu X, Zhai T, Li D, Zhou H (2013) Li3VO4: A Promising Insertion Anode Material for Lithium-Ion Batteries. Adv Energy Mater 3:428–432
Kim WT, Jeong YU, Lee YJ, Kim YJ, Song JH (2013) Synthesis and lithium intercalation properties of Li3VO4 as a new anode material for secondary lithium batteries. J Power Sources 244:557–560
Liang ZZ, Lin ZP, Zhao YM (2015) New understanding of Li3VO4/C as potential anode for Li-ion batteries: Preparation, structure characterization and lithium insertion mechanism. J Power Sources 274:345–354
Venkatesh G, Pralong V, Lebedev OI, Caignaert V, Bazin P, Raveau B (2014) Amorphous sodium vanadate Na1.5 + y VO3, a promising matrix for reversible sodium intercalation. Electrochemistry Communications 40:100–102
Ni SB, Ma JJ, Zhang JC, Yang XL (2014) Preparation method of lithium-ion battery negative electrode material. China patent:CN104393241
Larson AC, Von Dreele RB (2004) VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. Los Alamos National Laboratory Report LAUR, pp 86-748
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Applied Crystallography 34:210–213
Mendialdua J, Casonova R, Barbaux Y (1995) XPS studies of V2O5, V6O13, VO2 and V2O3. J Electron Spectroscopy and Related Phenomena 71:249–261
Cameron M, Sueno S, Prewitt CT, Papike JJ (1973) High-Temperature Grystal Ghemistry of Acmite, Diopside, Hedenbergite, Jadeite, Spodumene, and Ureyite. American Mineralogist 58:594–618
Zhang WK, Zhou XZ, Tao XY, Huang H, Gan YP, Wang CT (2010) In situ construction of carbon nano-interconnects between the LiFePO4 grains using ultra low-cost asphalt. Electrochim Acta 55:2592–2596
Rui XH, Li C, Chen CH (2009) Synthesis and characterization of carbon-coated Li3V2(PO4)3 cathode materials with different carbon sources. Electrochim Acta 54:3374–3380
Zhang D, Popov BN, White RE (1998) Electrochemical investigation of CrO2.65 doped LiMn2O4 as a cathode material for lithium-ion batteries. J Power Sources 76:81–90
Liu XD, Zhao YM (2015) Synthesis of Carbon-coated Nanoplate α-Na2MoO4 and its Electrochemical Lithiation Process as Anode Material for Lithium-ion Batteries. Electrochim Acta 154:94–101
Sharma Y, Sharma N, Rao GVS, Chowdari BVR (2008) Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Electrochim Acta 179:587–597
Kang YM, Song MS, Kim JH, Kim HS, Park MS, Lee JY, Liu HK, Dou SX (2005) A study on the charge-discharge mechanism of Co3O4 as an anode for the Li ion secondary battery. Electrochim Acta 50:3667–3673
Ryu JH, Kim JW, Sung YE, Oh SM (2004) Failure Modes of Silicon Powder Negative Electrode in Lithium Secondary Batteries. Electrochem Solid-State Lett 7:A306–A309
Jung YS, Lee KT, Ryu JH, Im D, Oh SM (2005) Sn-Carbon Core-Shell Powder for Anode in Lithium Secondary Batteries. J Electrochem Soc 152:A1452–A1457
Jung YS, Lee KT, Oh SM (2007) Si-carbon core-shell composite anode in lithium secondary batteries. Electrochim Acta 52:7061–7076
Acknowledgments
This work was funded by NSFC Grant (nos. 51372089, 51172077, and 51373205) supported through NSFC Committee of China, and the Foundation of (no. 2014ZB0014) supported through the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, X., Zhao, Y., Dong, Y. et al. A promising sol-gel method to synthesize NaVO3 as anode material for lithium ion batteries. J Solid State Electrochem 20, 1803–1812 (2016). https://doi.org/10.1007/s10008-016-3188-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3188-5