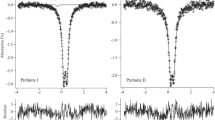
Abstract
Although the intercalation of Fe3+ into layered phyllosicilicates—especially into smectites—attracted much attention in the past two decades, the information about Fe2+ loaded phyllosilicates is sparse. Here we present an investigation of the Fe2+ exchanged vermiculites from Santa Olalla and Ojén (Andalusia, Spain) by means of Mössbauer spectroscopy. The room temperature Mössbauer spectra are very similar to those of the starting compounds (Na forms) except for a decrease of the contribution of structural Fe3+ and a concomitant increase of the contribution of Fe2+ sites, indicating an internal redox process. The extent of this redox reaction is different for the two vermiculites. Thus, the intercalated Fe2+ acts as an electron mediator from the external medium to the structural Fe3+ ions. A new component attributable to intercalated Fe2+ is practically invisible in the room temperature Mössbauer spectra, but increases strongly and continuously during cooling to 4.2 K, where it is the dominant feature of the Mössbauer patterns. At 4.2 K, its quadruple splitting amounts to 3.31 mm/s, which is in excellent agreement with the quadrupole slitting of Fe2+ coordinated to six water molecules in a highly symmetric octahedral arrangement. The strong decrease of the Mössbauer–Lamb factor of this component with increasing temperature indicates a weak bonding of the Fe2+ in the interlayer space.





Similar content being viewed by others
References
Rigthor EG, Tzou MS, Pinnavaia TJ (1991) J Catal 130:29
Gil A, Gandía LM, Vicente MA (2000) Catal Rev Sci Eng 42:145
Manju M, Sugunan S (2005) React Kinet Catal 85:37
Zhoua CH, Tonga DS, Baoa M, Dub ZX, Gea ZH, Li XN (2008) Top Catal 39:213
Oates JM (1984) Clays Clay Miner 37:49
Moreale A, Cloos P, Badot C (1985) Clay Miner 20:29
Helson JA, Goodman BA (1983) Clay Miner 18:117
Goodman BA, Helsen JA, Langouche G (1988) Hyperfine Interact 41:799
Ramírez-Valle V, Lerf A, Wagner FE, Poyato J, Pérez-Rodríguez JL (2008) Clay Miner 43:487
Kamei G, Oda C, Mitsui S, Shibata M, Shinozaki T (1999) Eng Geol 54:15
Kozai N, Adachi Y, Kawamura S, Inada K, Kozaki T, Sato S, Ohashi H, Ohnuki T, Banba T (2001) J Nucl Sci Techn 38:1141
Charlet L, Tournassat C (2005) Aquatic Geochem 11:115
Géhin A, Grenèche JM, Tournassat C, Brendlé J, Rancourt DG, Charlet L (2007) Geochim Cosmochim Acta 71:863
Stucki JW, Goodman BA, Schwertmann U (1988) Iron in soils and clay minerals. Reidel, Dordrecht
Fischer WR (1988) In: Stucki JW, Goodman BA, Schwertmann U (eds) Iron in soils and clay minerals, chapt. 20. Reidel, Dordrecht, p 715
Stucki JW (2006) in Bergaya F. Theng BKG, Lagaly G, (eds.) Handbook of clay science, Elsevier, Vol. 1, chapt. 8, p. 423
Stanjek H, Marchel C (2008) Clay Miner 43:62
Komadel P, Grygar T, Mehner H (1998) Clay Miner 33:593
Bailey SW (1988) In: Bailey SW (ed) Reviews inmMineralogy, Vol. 19. Mineralogical Society, Washington D.C., p 347
Laird J (1988) In: Bailey SW (ed) Reviews in mineralogy, Vol. 19. Mineralogical Society, Washington D.C., p 405
Gould K, Pe-Piper G, David JW, Piper DJW (2910) Sedimentology 57:587
Sandström B, Annersten H, Tullborg EL (2010) Int J Earth Sci 99:1
Poyato J, Pérez-Rodríguez JL, Ramírez-Valle V, Lerf A, Wagner FE (2009) Ultrasonics Sonication 16:570
Justo Erbez AJ (1984) Estudio fisicoquímico y mineralógico de vermiculitas de Andalucía y Badajoz. Ph.D. Thesis, Sevilla
Wagner FE, Lerf A, Poyato Ferrera J, Justo A, Perez Rodriguez JL (2001) In: Rammelmair D, Mederer J, Oberthür T, Heimann RB, Pettinghaus H (eds) Applied mineralogy, Vol. 2. Balkema, Rotterdam, p 927
Lerf A, Wagner FE, Poyato J (2001) Solid State Ionics 141–142:479
Wagner FE, Wagner U (2004) Hyperfine Interact 154:35
De Grave E, van Alboom A (1991) Phys Chem Miner 18:337
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Robert Schöllhorn at the occasion of his 75th birthday
Rights and permissions
About this article
Cite this article
Lerf, A., Wagner, F.E., Poyato, J. et al. Intercalation and dynamics of hydrated Fe2+ in the vermiculites from Santa Olalla and Ojén. J Solid State Electrochem 15, 223–229 (2011). https://doi.org/10.1007/s10008-010-1171-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1171-0