Abstract
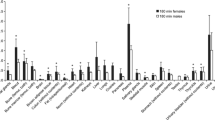
The aim of the present study was to label EGCG with 125I and to determine its radiopharmaceutical potential in mice. EGCG was labeled with 125I using the iodogen method. The labeling yield and the radiochemical purity of 125I–EGCG were determined by radio thin-layer chromatography (RTLC). The Labeling yield was approximately 89.4 %. The radiochemical purity was approximately 96.4 %. The biodistribution studies of the labeled compound (specific activity; 0.47 TBq/μg) were performed in male Kunming mice. The uptakes of 125I–EGCG in some organs were determined at different time after injection to the mice. The radioactivity in each organ was counted and the percentage of injected activity per gram of tissue weight (%ID/g) for each organ and blood was calculated. Incorporation of radioactivity in the various tissue/organ was confirmed by microautoradiography. 125I–EGCG uptake in the stomach and salivary gland was higher than other organ/tissue. The black silver grains was concentrated in the nucleus, cytoplasm, intercellular substance and capillaries of that various organs, and its unevenly distributed. Thus, 125I–EGCG may be radiopharmaceutical for the imaging of the stomach and salivary gland.



Similar content being viewed by others
References
El-shahawi MS, Hamza A, Bahaffi SO, Al-Sibaai AA, Abduljabbar TN (2012) Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem 134(4):2268–2275
Chen WW, Qin GY, Zhang T, Feng WY (2012) In vitro drug metabolism of green tea catechins in human, monkey, dog, rat and mouse hepatocytes. Drug Metab Lett 6(2):73–93
Lu C, Zhu W, Shen CL, Gao W (2012) Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS One 7(6):e 38332. doi:10.1371/0038332
Jagdeo J, Brody N (2011) Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J Drug Dematol 10(7):753–761
Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ (2011) Anti-inflammatory and anti-oxidative effects of green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis 17:533–542
Abib RT, Peres KC, Barbosa AM, Peres TV, Bernardes A, Zimmermann LM, Quincozes-Santos A, Fiedler HD, Leal RB, Farina M, Gottfried C (2011) Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem Toxicol 49(10):2618–2623
Suzuki Y, Miyoshi N, Isemura M (2012) Health-promoting effects of green tea. Proc Jpn Acad B 88(3):88–101
Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Faria JB, Faria JM (2013) Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci 54(2):1325–1336
Thring TS, Hili P, Naughton DP (2009) Anti-collagenase, anti-elastase, anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med 9:27. doi:10.1186/1472-6882-9-27
Tipoe GL, Leung TM, Hung MW, Fung ML (2007) Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord 7(2):135–144
Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, Gopalakrishna R (2012) Green tea polyphenols precondition against cell death induced by oxygen–glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase C. J Biol Chem 287(41):34694–34708
Park SY, Jeong YJ, Kim SH, Jung JY, Kim WJ (2013) Epigallocatechin gallate protects against nitric oxide-induced apoptosis via scavenging ROS and modulating the Bcl-2 family in human dental pulp cells. J Toxicol Sci 38(3):371–378
Clifford MN, Van der Hooft JJ, Crozier A (2013) Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr. doi:10.3945/113.058958
Nakagawa K, Nakayama K, Nakamura M, Sookwong P, Tsuduki T, Niino H, Kimura F, Miyazawa T (2009) Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci Biotechnol Biochem 73(9):2014–2017
Yang CS, Pan E (2012) The effects of green tea polyphenols on drug metabolism. Expert Opin Drug Metab Toxicol 8(6):677–689
Feng WY (2006) Metabolism of green tea catechines: an overview. Curr Drug Metab 7(7):755–809
Dona M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S (2003) Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol 170(8):4335–4341
Kumar P, Mc Quarrie SA, Zhou Y, McEwan AJ, Wiebe LI (2005) [131I] iodoazomycin arabinoside for low-dose-rate isotope radio therapy: radiolabeling, stability, long-term whole-body clearance and radiation dosimetry estimates in mice. Nucl Med Biol 32(6):647–653
Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS (2003) Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun 310(1):222–227
Okabe M, Suganuma M, Hayashi M, Sueoka E, Komori A, Fujiki H (1997) Mechanisms of growth inhibition of human lung cancer cell line, PC-9 by tea polyphenols. Jpn J Cancer Res 88(7):639–643
Hackett AM (1986) The metabolism of flavonoid compounds in mammals. Prog Clin Biol Res 213:177–194
Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H (1998) Wide distribution of 3H-(−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19(10):1771–1776
Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N (2001) Metabolic fate of (−)-[4-(3)H] epigallocatechin gallate in rats after oral administration. J Agric Food Chem 49(8):4102–4112
Kohri T, Nanjo F, Suzuki M, Seto R, Matsumoto N, Yamakawa M, Hojo H, Hara Y, Desai D, Amin S, Conaway CC, Chung FL (2001) Synthesis of (−)-[4-3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J Agric Food Chem 49(2):1042–1048
Acknowledgments
This work was supported by the Liaoning province Natural Science Foundation of China (Grant no: 20092118).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diao, Y., Zhao, W., Li, Y. et al. Radiolabeling of EGCG with 125I and its biodistribution in mice. J Radioanal Nucl Chem 301, 167–173 (2014). https://doi.org/10.1007/s10967-014-3124-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3124-z