Abstract
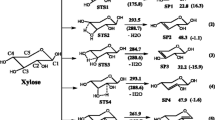
O-acetyl-4-O-methylglucurono-xylan is selected as a model compound because of its abundant O-acetyl and 4-O-methylglucuronic acid groups as side chains of hemicellulose. The detailed decomposition pathways of O-acetyl-4-O-methylglucurono-xylan are investigated by using density functional theory (DFT) and transition state theory. In addition, the possible pyrolysis pathways of 4-O-methylglucuronic acid, based on the Mayer bond order values, are predicted. The results indicate that the most energetically favored initial reaction of O-acetyl-4-O-methylglucurono-xylan is the cleavage of the 4-O-methylglucuronic acid unit. Furfural can be obtained through the ring-opening of 4-O-methylglucuronic acid in three different pathways. The O-methyl group is predominantly responsible for the generation of CH3OH. In addition, the formation pathways of a special furan-derived product 2-hydroxymethylene-tetrahydrofuran-3-one are first validated by DFT calculation. The rate-determining steps to form 2-hydroxymethylene-tetrahydrofuran-3-one are the cyclization reaction and enol–keto tautomerization. Anhydroxylopyranose and dianhydroxylopyranose can be produced through intramolecular dehydration and glycosidic bond cleavage reactions.

The main pyrolysis products distribution of O-acetyl-4-O-methylglucurono-xylan






















Similar content being viewed by others
References
Cai W, Liu R, He Y, Chai M, Cai J (2018) Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Process Technol 171:308–317
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89:913–933
Wu Y, Wu S, Zhang H, Xiao R (2018) Cellulose-lignin interactions during catalytic pyrolysis with different zeolite catalysts. Fuel Process Technol 179:436–442
Bledzki AK, Gassan J (1999) Composites reinforced with cellulose based fibres. Prog Polym Sci 24:221–274
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Lin H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206
Peng F, Peng P, Xu F, Sun R-C (2012) Fractional purification and bioconversion of hemicelluloses. Biotechnol Adv 30:879–903
Wang S, Ru B, Lin H, Sun W (2015) Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods. Fuel 150:243–251
Shen DK, Gu S, Bridgwater AV (2010) Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J Anal Appl Pyrolysis 87:199–206
Shen DK, Gu S, Bridgwater AV (2010) The thermal performance of the polysaccharides extracted from hardwood: cellulose and hemicellulose. Carbohydr Polym 82:39–45
Wang S, Ru B, Dai G, Sun W, Qiu K, Zhou J (2015) Pyrolysis mechanism study of minimally damaged hemicellulose polymers isolated from agricultural waste straw samples. Bioresour Technol 190:211–218
Huang X, Cheng D-g, Chen F, Zhan X (2016) Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: a density functional theory study. Renew Energy 96:490–497
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Chen J, Wang S, Lu L, Zhang X, Liu Y (2018) Improved catalytic upgrading of simulated bio-oil via mild hydrogenation over bimetallic catalysts. Fuel Process Technol 179:135–142
Dai G, Wang S, Zou Q, Huang S (2018) Improvement of aromatics production from catalytic pyrolysis of cellulose over metal-modified hierarchical HZSM-5. Fuel Process Technol 179:319–323
Lu Q, Guo H-q, Zhou M-x, Cui M-s, Dong C-q, Yang Y-p (2018) Selective preparation of monocyclic aromatic hydrocarbons from catalytic cracking of biomass fast pyrolysis vapors over Mo 2 N/HZSM-5 catalyst. Fuel Process Technol 173:134–142
Zhou X, Li W, Mabon R, Broadbelt LJ (2018) A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ Sci 11:1240–1260
Zhao C, Jiang E, Chen A (2016) Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J Energy Inst 90:902–913
Wang S, Ru B, Lin H, Luo Z (2013) Degradation mechanism of monosaccharides and xylan under pyrolytic conditions with theoretic modeling on the energy profiles. Bioresour Technol 143:378–383
Wang S-r, Liang X-j (2013) Mechanism of xylan pyrolysis by Py-GC/MS. Chem Res Chin Univ 29:782–787
Huang J, He C, Wu L, Tong H (2016) Thermal degradation reaction mechanism of xylose: a DFT study. Chem Phys Lett 658:114–124
Li Z, Liu C, Xu X, Li Q (2017) A theoretical study on the mechanism of xylobiose during pyrolysis process. Comput Theor Chem 1117:130–140
Patwardhan PR, Brown RC, Shanks BH (2011) Product distribution from the fast pyrolysis of hemicellulose. ChemSusChem 4:636–643
Zhang J, Choi YS, Yoo CG, Kim TH, Brown RC, Shanks BH (2015) Cellulose–hemicellulose and cellulose–lignin interactions during fast pyrolysis. ACS Sustain Chem Eng 3:293–301
Hosoya T, Kawamoto H, Saka S (2007) Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J Anal Appl Pyrolysis 78:328–336
Mayer I (1985) Bond orders and valences in the SCF theory: a comment. Theor Chim Acta 67:315–322
Frisch M, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Zheng G, Bloino J, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Vreven T, Nakai H, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene KJM, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts SRR, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Cheminform 41:157–167
Parthasarathi R, Romero RA, Redondo A, Gnanakaran S (2015) Theoretical study of the remarkably diverse linkages in lignin. J Phys Chem Lett 2:2660–2666
Barreto PCRP, Vilela AFA, Gargano R (2003) A simple program to determine the reaction rate and thermodynamic properties of reacting system. J Mol Struct THEOCHEM 639:167–176
Gokhale AA, Kandoi S, Greeley JP, Mavrikakis M, Dumesic JA (2004) Molecular-level descriptions of surface chemistry in kinetic models using density functional theory. Chem Eng Sci 59:4679–4691
Lu Q, Tian H-y, Hu B, Jiang X-y, Dong C-q, Yang Y-p (2016) Pyrolysis mechanism of holocellulose-based monosaccharides: the formation of hydroxyacetaldehyde. J Anal Appl Pyrolysis 120:15–26
Mayer I (1985) Charge, bond order and valence in the AB initio SCF theory. Chem Phys Lett 97:270–274
Rong C, Ding X, Zhu Y, Li Y, Wang L, Qu Y, Ma X, Wang Z (2012) Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts. Carbohydr Res 350:77
Huang J, Liu C, Tong H, Li W, Wu D (2012) Theoretical studies on pyrolysis mechanism of xylopyranose. Comput Theor Chem 1001:44–50
Paine JB, Pithawalla YB, Naworal JD (2008) Carbohydrate pyrolysis mechanisms from isotopic labeling. J Anal Appl Pyrolysis 82:10–41
Peng Y, Wu S (2011) Fast pyrolysis characteristics of sugarcane bagasse hemicellulose, cellulose. Chem Technol 45:605–612
Yang H, Yan R, Chen H, Dong HL, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788
Acknowledgments
This work is supported by National Natural Science Foundation of China (No. 51576019) and the Graduate Research and Innovation Foundation of Chongqing, China (No. CYS18040).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 7096 kb)
Rights and permissions
About this article
Cite this article
Wu, J., Liu, C. & Li, Q. Thermal decomposition mechanism of O-acetyl-4-O-methylglucurono-xylan. J Mol Model 25, 234 (2019). https://doi.org/10.1007/s00894-019-4117-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4117-1