Abstract
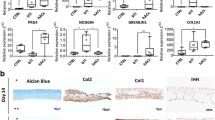
Multilineage potential of progenitor cells from periosteum is well established, but conditions for differentiation within their native niche are unclear. We evaluated at cellular and molecular levels whether chondrogenesis of periosteal progenitor cells is promoted spontaneously or by growth-factor mixture (GFM) application when transferring periosteum–bone cylinders into cartilage defects. Osteochondral defects in the patellar groove of minipigs were filled with periosteum–bone cylinders and randomly supplemented with GFM. Neochondrogenesis was characterized by histology, immunohistology, and quantitative gene expression analysis. According to morphology and glycosaminoglycan accumulation, spontaneous neocartilage formation occurred in the cambium layer already at 6 weeks, increased after 12 weeks, but declined until 52 weeks, independent of GFM. Multiple cartilage differentiation markers were induced after transfer. Expression of aggrecan, COMP, decorin, and Col10a1 increased significantly within 52 weeks. Sox 9 and Col2a1 mRNA levels were elevated at 6 versus 52 weeks in the GFM group and resulted in higher collagen type II protein accumulation. Neochondrogenesis was promoted in lower periosteum layers by transfer of periosteum–bone plugs into a joint, and collagen type II protein deposition was enhanced by GFM. The final tissue subsumed typical features of periosteum and fibrocartilage but lacked an intact tide mark and features of hyaline cartilage desired for cartilage repair.








Similar content being viewed by others
References
Aigner T, Stoss H, Weseloh G, Zeiler G, von der Mark K (1992) Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol 62:337–345
Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L (2001) Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum 44:2777–2789
Atkinson BL, Fantle KS, Benedict JJ, Huffer WE, Gutierrez-Hartmann A (1997) Combination of osteoinductive bone proteins differentiates mesenchymal C3H/10T1/2 cells specifically to the cartilage lineage. J Cell Biochem 65:325–339
Boden SD, Schimandle JH, Hutton WC (1995) Lumbar intertransverse-process spinal arthrodesis with use of a bovine bone-derived osteoinductive protein. A preliminary report. J Bone Joint Surg Am 77:1404–1417
Boden SD, Grob D, Damien C (2004) Ne-Osteo bone growth factor for posterolateral lumbar spine fusion: results from a nonhuman primate study and a prospective human clinical pilot study. Spine 29:504–514
Damien CJ, Grob D, Boden SD, Benedict JJ (2002) Purified bovine BMP extract and collagen for spine arthrodesis: preclinical safety and efficacy. Spine 27:S50–S58
Draenert K, Dick Y (1988) A new procedure for bone biopsies and cartilage and bone transplantation. Sandorama III-IV:33–40
Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ (1996) Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett 380:17–20
Gotterbarm T, Reitzel T, Schneider U, Voss HJ, Stofft E, Breusch SJ (2003) Integration of periosteum covered autogenous bone grafts with and without autologous chondrocytes. An animal experiment using the Gottinger minipig. Orthopade 32:65–73
Ham AW (1930) A histological study of the early phases of bone repair. J Bone Joint Surg 12:827–844
Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM (1996) Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13:291–300
Ito Y, Fitzsimmons JS, Sanyal A, Mello MA, Mukherjee N, O’Driscoll SW (2001) Localization of chondrocyte precursors in periosteum. Osteoarthritis Cartilage 9:215–223
Korkala OL, Kuokkanen HO (1995) Autoarthroplasty of knee cartilage defects by osteoperiosteal grafts. Arch Orthop Trauma Surg 114:253–256
Maroudas A (1980) Physicochemical properties of articular cartilage. Tunbridge Wells, UK
Miura Y, Parvizi J, Fitzsimmons JS, O’Driscoll SW (2002) Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro:a preliminary report. J Bone Joint Surg Am 84-A:793–799
Mizuta H, Matsui N, Sanyal A (1998) Expression of TGF-[beta] type I and type II receptors during periosteal chondrogenesis in vitro. Trans Orthop Res Soc 23:511
Nakahara H, Bruder SP, Goldberg VM, Caplan AI (1990) In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop 259:223–232
O’Driscoll SW (1999) Articular cartilage regeneration using periosteum. Clin Orthop 387:S186–S203
O’Driscoll SW (2001) Technical considerations in periosteal grafting for osteochondral injuries. Clin Sports Med 20:379–402, vii
O’Driscoll SW, Fitzsimmons JS (2000) The importance of procedure specific training in harvesting periosteum for chondrogenesis. Clin Orthop 380:269–278
O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS (2001) The chondrogenic potential of periosteum decreases with age. J Orthop Res 19:95–103
Poussa M, Rubak J, Ritsila V (1981) Differentiation of the osteochondrogenic cells of the periosteum in chondrotrophic environment. Acta Orthop Scand 52:235–239
Rubak JM, Poussa M, Ritsila V (1982) Chondrogenesis in repair of articular cartilage defects by free periosteal grafts in rabbits. Acta Orthop Scand 53:181–186
Sanyal A, Oursler MJ, Clemens VR, Fukumoto T, Fitzsimmons JS, O’Driscoll SW (2002) Temporal expression patterns of BMP receptors and collagen II (B) during periosteal chondrogenesis. J Orthop Res 20:58–65
Strayhorn CL, Garrett JS, Dunn RL, Benedict JJ, Somerman MJ (1999) Growth factors regulate expression of osteoblast-associated genes. J Periodontol 70:1345–1354
van Susante JL, Wymenga AB, Buma P (2003) Potential healing benefit of an osteoperiosteal bone plug from the proximal tibia on a mosaicplasty donor-site defect in the knee. An experimental study in the goat. Arch Orthop Trauma Surg 123:466–470
Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W (2003) Cartilage-like gene expression in differentiated human stem cell spheroids:a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 48:418–429
Wozney JM (2002) Overview of bone morphogenetic proteins. Spine 27:S2–S8
Zarnett R, Salter RB (1989) Periosteal neochondrogenesis for biologically resurfacing joints: its cellular origin. Can J Surg 32:171–174
Acknowledgements
We thank Kathrin Götzke, Regina Föhr, and Valeska Link for expert technical assistance, Helga Lorenz for help with animal surgery, and Sven Schneider for statistical analysis. This work was funded by Centerpulse Biologics and the Research Fund of the Orthopaedic University Hospital of Heidelberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, M., Gotterbarm, T., Gruettgen, A. et al. Molecular characterization of spontaneous and growth-factor-augmented chondrogenesis in periosteum–bone tissue transferred into a joint. Histochem Cell Biol 123, 447–456 (2005). https://doi.org/10.1007/s00418-005-0775-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0775-4