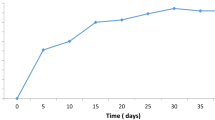
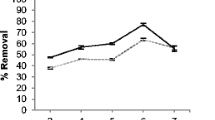
Abstract
Posidonia oceanica (L.), a marine biomass, has been used as an effective and efficient biosorbent for the removal of Cd(II) from aqueous media. The physico-chemical properties of biosorbent were investigated by elemental analysis, BET surface area, FT-IR, SEM and EDX methods before and after adsorption. Batch adsorption experiments were carried out to investigate the effects of solution pH, dosage of biosorbent, contact time and temperature. The biosorbent exhibited the maximum uptake of 58.82 mg/g under the optimal adsorption condition. Kinetics experiments indicated that the pseudo-second-order model displayed the best correlation with adsorption kinetics data. Besides, experimental data could be better described by the Langmuir isotherm model. Desorption experiments were carried out to explore the feasibility of regenerating the biosorbent. The regeneration efficiency was 96.03 % using desorption agent of 0.2 M HCl. The thermodynamic parameters (ΔH 0, ΔS 0 and ΔG 0) of the cadmium ion uptake onto P.O indicated that the process is endothermic and proceeds spontaneously. The findings of the present study indicate that P.O can be successfully used for separation of Cd(II) from aqueous solutions. The results suggested that the adsorbent is promising for use as an effective and economical adsorbent for Cd(II) ions removal.







Similar content being viewed by others
Change history
15 January 2018
The authors are retracting this article (Krika et al., 2015) because Figure 2C and the text describing it are duplicated from a previously published article by Allouche et al. (2011). All authors agree to this retraction.
15 January 2018
The authors are retracting this article (Krika et al., 2015) because Figure 2C and the text describing it are duplicated from a previously published article by Allouche et al. (2011). All authors agree to this retraction.
References
Al-Degs YS, El-Barghouthi MI, Issa AA, Khraisheh MA, Walker GM (2006) Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Res 40:2645–2658
Chen JH, Ni JC, Liu QL, Li SX (2012) Adsorption behavior of Cd(II) ions on humic acid-immobilized sodium alginate and hydroxyl ethyl cellulose blending porous composite membrane adsorbent. Desalination 285:54–61
Coates J (2000) Interpretation of infrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. John Wiley & Sons Ltd., Chichester
Febrianto J, Kosasih AN, Sunarso J, Jua Y, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Freundlich H (1906) Adsorption in solution. Phys Chem Soc 40:1361–1368
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Holan ZR, Volesky B, Prasetyo I (1993) Biosorption of cadmium by biomass of marine algae. Biotechnol Bioeng 41:819–825
Kellner RJ, Mermet JM, Otto M (1998) Analytical chemistry. Wiley/VCH Verlag GmbH Press, New York
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1368
Laus R, Costa TG, Szpoganicz B, Fávere VT (2010) Adsorption and desorption of Cu(II), Cd(II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin–triphosphate as the adsorbent. J Hazard Mater 183:233–241
Leyva-Ramos R, Rangel-Mendez JR, Mendoza-Barron J, Fuentes-Rubio L, Guerrero-Coronado RM (1997) Adsorption of cadmium(II) from aqueous solution onto activated carbon. Water Sci Technol 35:205–211
Li Q, Chai L, Yang Z, Wang Q (2009) Kinetics and thermodynamics of Pb(II) adsorption onto modified spent grain from aqueous solutions. Appl Surf Sci 255:4298–4303
Liu CX, Bai RB (2006) Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes. J Membr Sci 284:313–322
Lua D, Caob Q, Cao X, Luo F (2009) Removal of Pb(II) using the modified lawny grass: mechanism, kinetics, equilibrium and thermodynamic studies. J Hazard Mater 166:239–247
Mansour MS, Ossman ME, Farag HA (2011) Removal of Cd(II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination 272:301–305
Nomanbhay SM, Palanisamy K (2005) Removal of heavy metals from industrial wastewater using chitosan coated oil palm shell charcoal. Electron J Biotechnol 8:43–53
Puranik PR, Paknikar KM (1997) Biosorption of lead and zinc from solutions using Streptoverticillium cinnamoneum waste biomass. J Biotechnol 55:113–124
Reddy DHK, Ramana DKV, Seshaiah K, Reddy AVR (2011) Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 268:150–157
Reddy DHK, Lee SM, Seshaiah K (2012) Removal of Cd(II) and Cu(II) from aqueous solution by agro biomass: equilibrium, kinetic and thermodynamic studies. Environ Eng Res 17:125–132
Redlich OJ, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024
Saeed A, Iqbal M (2003) Bioremoval of cadmium from aqueous solution by black gram husk (Cicer arietinum). Water Res 37:3472–3480
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of Pb(II) and Cd(II) removal from aqueous solution onto colemanite ore waste. Desalination 249:260–266
Tsezos M (2001) Biosorption of metals: the experience accumulated and the outlook for technology development. Hydrometallurgy 59:241–243
Valdman E, Leite SGF (2000) Biosorption of Cd, Zn and Cu by Sargassum sp. waste biomass. Bioprocess Eng 22:171–173
Wang SB, Terdkiatburana T, Tad MO (2008) Single and co-adsorption of heavy metals and humic acid on fly ash. Sep Purif Technol 58:353–358
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–59
Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174:137–143
Acknowledgments
Special thanks to Dr. Eric Guibal for his invaluable contribution in the experimental work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are retracting this article (Krika et al., 2015) because Figure 2C and the text describing it are duplicated from a previously published article by Allouche et al. (2011). All authors agree to this retraction.
Allouche F-N, Mameri N, Guibala E (2011) Pb(II) biosorption on Posidonia oceanica biomass. Chem Eng J 168:1174–1184
Krika, F Azzouz N, Ncibi MC (2015) Adsorptive removal of cadmium from aqueous media using Posidonia oceanica biomass: equilibrium, dynamic and thermodynamic studies. Int J Environ Sci Technol 12:983–994
A correction to this article is available online at https://doi.org/10.1007/s13762-017-1595-5.
An erratum to this article is available at https://doi.org/10.1007/s13762-017-1595-5.
About this article
Cite this article
Krika, F., Azzouz, N. & Ncibi, M.C. RETRACTED ARTICLE: Adsorptive removal of cadmium from aqueous media using Posidonia oceanica biomass: equilibrium, dynamic and thermodynamic studies. Int. J. Environ. Sci. Technol. 12, 983–994 (2015). https://doi.org/10.1007/s13762-013-0483-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0483-x