Abstract
Introduction
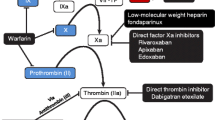
EP217609 is a representative of a new class of synthetic parenteral anticoagulants with a dual mechanism of action. It combines in a single molecule a direct thrombin inhibitor and an indirect factor Xa inhibitor. EP217609 can be neutralized by a specific antidote avidin, which binds to the biotin moiety of EP217609.
Purpose
The primary objective was to assess the neutralization of EP217609 by avidin in healthy subjects. Secondary objectives were to define the optimal avidin monomer/EP217609 molar ratio to achieve an adequate neutralization of EP217609 and to assess the safety and tolerability of EP217609 and avidin.
Methods
Healthy subjects (n = 36) were randomized to a 3 by 3 replicated Latin square design between 3 EP217609 doses (4, 8, 12 mg) and 3 avidin monomer/EP217609 molar ratios (1:1; 2:1; 3:1). EP217609 was administered as a single intravenous bolus, and avidin as a 30-min intravenous infusion, starting 90 min after EP217609 administration.
Results
Overall, EP217609 and avidin were well tolerated. One subject experienced a benign and transient typical pseudo-allergic reaction. The administration of EP217609 resulted in dose-dependent increases in pharmacodynamic markers. Avidin triggered a rapid and irreversible neutralization of EP217609 without rebound effect. Adequate neutralization of the anticoagulant activity was achieved with both 2:1 and 3:1 avidin monomer/EP217609 molar ratios. All safety parameters did not show any treatment-emergent clinically relevant changes or abnormalities in any dose group.
Conclusions
These results will allow further investigation in patients requiring a neutralizable anticoagulant as those undergoing cardiac surgery.
Study registration
EudraCT number 2010-020216-10.






Similar content being viewed by others
References
Olson ST, Swanson R, Petitou M (2012) Specificity and selectivity profile of EP217609: a new, neutralizable, dual-action anticoagulant that targets thrombin and factor Xa. Blood 119(10):2187–2195
Petitou M, Nancy-Portebois V, Dubreucq G, Motte V, Meuleman D, de Kort M, van Boeckel CAA, Vogel GMT, Wisse JAJ (2009) From heparin to EP217609: the long way to a new pentasaccharide-based neutralisable anticoagulant with an unprecedented pharmacological profile. Thromb Haemost 102:804–810
Gueret P, Combe S, Krezel C, Fuseau E, van Giersbergen PL, Petitou M, Neuhart E (2016) First in man study of EP217609, a new long-acting, neutralisable parenteral antithrombotic with a dual mechanism of action. Eur J Clin Pharmacol. doi:10.1007/s00228-016-2077-2
Snyder-Ramos SA, Möhnle P, Weng YS, Böttiger BW, Kulier A, Levin J, Mangano DT, Investigators of the Multicenter Study of Perioperative Ischemia; MCSPI Research Group (2008) The ongoing variability in blood transfusion practices in cardiac surgery. Transfusion 48(7):1284–1299
Yavari M, Becker RC (2008) Anticoagulant therapy during cardiopulmonary bypass. J Thromb Thrombolysis 26:218–228
Merry AF (2007) Focus on thrombin: alternative anticoagulants. Semin Cardiothorac Vasc Anesth 11(4):256–260
Smedira NG, Dyke CM, Koster A, Jurmann M, Bhatia DS, Hu T, McCarthy HL 2nd, Lincoff AM, Spiess BD, Aronson S (2006) Anticoagulation with bivalirudin for off-pump coronary artery bypass grafting: the results of the EVOLUTION-OFF study. J Thorac Cardiovasc Surg 131(3):686–692
Dyke CM, Smedira NG, Koster A, Aronson S, McCarthy HL 2nd, Kirshner R, Lincoff AM, Spiess BD (2006) A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: the EVOLUTION-ON study. J Thorac Cardiovasc Surg 131(3):533–539
Paparella D, Brister SJ, Buchanan MR (2004) Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med 30:1873–1881
Christensen MC, Krapf S, Kempel A, von Heymann C (2009) Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg 138(3):687–693
Gummert JF, Funkat A, Beckmann A, Schiller W, Hekmat K, Ernst M, Haverich A (2009) Cardiac surgery in Germany during 2008. A report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 57(6):315–323
Scott BH, Seifert FC, Grimson R (2008) Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Ann Card Anaesth 11:15–19
Brown PP, Kugelmass AD, Cohen DJ, Reynolds MR, Culler SD, Dee AD, Simon AW (2008) The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg 85(6):1980–1986
Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD (2007) Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 116(22):2544–2552
Juneja R, Mehta Y (2008) Blood transfusion is associated with increased resource utilisation, morbidity, and mortality in cardiac surgery. Ann Card Anaesth 11(2):136–137
Nashef SAM, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R, the EuroSCORE study group (1999) European system for cardiac operative risk evaluation (EuroSCORE). Eur J Card Thor Surg 16:9–13
Pötzsch B, Hund S, Madlener K, Unkrig C, Müller-Berghaus G (1997) Monitoring of recombinant hirudin: assessment of a plasma-based ecarin clotting time assay. Thromb Res 86(5):373–383
Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Béguin S (2003) Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 33(1):4–15
Hemker HC, Al Dieri R, De Smedt E, Béguin S (2006) Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 96(5):553–561
Nowak G (2003) The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 33(4):173–183
Acknowledgments
The clinical part of the study was conducted at Biotrial, Rennes, France, with Marie-Claude Homery, acting as the principal investigator. Special thanks to Isabelle Gouin Thibault at University hospital Pontchaillou, Rennes, for the careful proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The study was funded by Endotis Pharma, Romainville, France. EN, CK and MP were full-time employees of Endotis Pharma at the time of study conduct and reporting. PG, SC, EF and PvG received financial compensation for their involvement in the study. All authors are fully responsible for content and editorial decisions regarding this paper.
Rights and permissions
About this article
Cite this article
Gueret, P., Combe, S., Krezel, C. et al. Neutralization of EP217609, a new dual-action FIIa/FXa anticoagulant, by its specific antidote avidin: a phase I study. Eur J Clin Pharmacol 73, 15–28 (2017). https://doi.org/10.1007/s00228-016-2143-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2143-9