Abstract
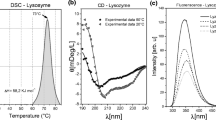
The formation of protein aggregates is important in many fields of life science and technology. The morphological and mechanical properties of protein solutions depend upon the molecular conformation and thermodynamic and environmental conditions. Non-native or unfolded proteins may be kinetically trapped into irreversible aggregates and undergo precipitation or gelation. Here, we study the thermal aggregation of lysozyme in neutral solutions. We characterise the irreversible unfolding of lysozyme by differential scanning calorimetry. The structural properties of aggregates and their mechanisms of formation with the eventual gelation are studied at high temperature by spectroscopic, rheological and scattering techniques. The experiments show that irreversible micron-sized aggregates are organised into larger clusters according to a classical mechanism of diffusion and coagulation, which leads to a percolative transition at high concentrations. At a smaller length scale, optical and atomic force microscopy images reveal the existence of compact aggregates, which are the origin of the aggregation irreversibility.









Similar content being viewed by others
Abbreviations
- SALS:
-
Small-angle light scattering
- DSC:
-
Differential scanning calorimetry
- AFM:
-
Atomic force microscopy
- OD:
-
Optical density
References
Arnaudov LN, de Vries R (2005) Thermally induced fibrillar aggregation of hen egg white lysozyme. Biophys J 88(1):515–526
Azuaga AI, Dobson CM, Mateo PL, Conejero-Lara F (2002) Unfolding and aggregation during the thermal denaturation of streptokinase. Eur J Biochem 269:4121–4133
Barnes HA, Hutton JF, Walters K (1989) An introduction to rheology. Elsevier, Amsterdam
Barone G, Giancola C, Verdoliva A (1992) Dsc studies on the denaturation and aggregation of serum albumins. Thermochim Acta 199:197–205
Baussay K, Bon CL, Nicolai T, Durand D, Busnel J-P (2004) Influence of the ionic strength on the heat-induced aggregation of the globular protein b-lactoglobulin at pH 7. Int J Biol Macromol 34:21–28
Bulone D, Fornili LG, Fornili SL, Lapis M, San Biagio PL (1994) Transputer-based upgrading of a differential scanning calorimeter. Meas Sci Technol 5:1443–1447
Cao A, Hu D, Lai L (2004) Formation of amyloid fibrils from fully reduced hen egg white lysozyme. Protein Sci 13:319–324
Carrotta R, Barthès J, Longo A, Martorana V, Manno M, Portale G, San Biagio PL (2007) Large size fibrillar bundles of the Alzheimer amyloid β-protein. Eur Biophys J 36:701–709
Carrotta R, Manno M, Giordano FM, Longo A, Portale G, Martorana V, San Biagio PL (2009) Protein stability modulated by a conformational effector: effects of trifluoroethanol on bovine serum albumin. Phys Chem Phys 11:4007–4018
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Clark A, Kavanagh G, Ross-Murphy S (2001) Globular protein gelation-theory and experiment. Food Hydrocolloids 15:383–400
Claudy P, Létoffé JM, Bayol A, Bonnet MC, Maurizot JC (1992) Denaturation versus pH of lysozyme and biosynthetic human growth hormone by differential scanning calorimetry and circular dichroism: a comparative study. Thermochim Acta 207:227–237
Dill KA (1990) Dominant forces in protein folding. Biochemistry 29(31):7133–7155
Ferry JD (1948) Protein gels. Adv Protein Chem 4:1–78
Ferry JD (1980) Viscoelastic properties of polymers. Wiley, New York
Flyvbjerg H, Jobs E, Leibler S (1996) Kinetics of self-assembling microtubules: an “inverse problem” in biochemistry. Proc Natl Acad Sci USA 93:5975–5979
Gosal W, Ross-Murphy SB (2000) Globular protein gelation. Curr Opin Colloid Interface Sci 5:188–194
Gray KA, Richardson TH, Kretz K, Short JM, Bartnek F, Knowles R, Kan L, Swanson P, Robertson DE (2001) Rapid evolution of reversible denaturation and elevated melting temperature in a microbial haloalkane dehalogenase. Adv Synth Catal 343:607–617
Hedoux A, Ionov R, Willart J-F, Lerbret A, Affouard F, Guinet Y, Descamps M, Prevost D, Paccou L, Danede F (2006) Evidence of a two-stage thermal denaturation process in lysozyme: a Raman scattering and differential scanning calorimetry investigation. J Chem Phys 124:014703
Kato A, Fujimoto K, Matsudomi N, Kobayashi K (1986) Protein flexibility and functional properties of heat-denatured ovalbumin and lysozyme. Agric Biol Chem 50:417–420
Krebs M, Wilkins D, Chung E, Pitkeathly M, Chamberlain A, Zurdo J, Robinson C, Dobson C (2000) Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the beta-domain. J Mol Biol 300:541–549
Krebs MRH, Devlin GL, Donald AM (2007) Protein particulates: another generic form of protein aggregation? Biophys J 92:1336–1342
Lashuel HA, LaBrenz SR, Woo L, Serpell LC, Kelly JW (2000) Protofilaments, filaments, ribbons, and fibrils from peptidomimetic self-assembly: implications for amyloid fibril formation and materials science. J Am Chem Soc 122:5262–5277
Manno M, Craparo EF, Podestà A, Bulone D, Carrotta R, Martorana V, Tiana G, San Biagio PL (2007) Kinetics of different processes in human insulin amyloid formation. J Mol Biol 366:258–274
Manno M, Xiao C, Bulone D, Martorana V, San Biagio PL (2003) Thermodynamic instability in supersaturated lysozyme solutions: effect of salt and role of concentration fluctuations. Phys Rev E 68:011904
Militello V, Vetri V, Leone M (2003) Conformational changes involved in thermal aggregation processes of bovine serum albumin. Biophys Chem 105:133–141
Mine Y, Noutomi T, Haga N (1990) Thermally induced changes in egg white proteins. J Agric Food Chem 38:2122–2125
Muschol M, Rosenberger F (1997) Liquid–liquid phase separation in supersaturated lysozyme solution and associated precipitate formation/crystallization. J Chem Phys 107:1953–1962
Navarra G, Giacomazza D, Leone M, Militello V, San Biagio PL (2009) Pre-aggregates characterization and viscoelastic studies of BSA cold gels induced by metal ions. Eur Biophys J doi:10.1007/s00249-008-0389-6
Nayakawa S, Nakamura R (1986) Optimization approaches to thermally induced egg white lysozyme gel. Agric Biol Chem 50:2039–2046
Nicolai T, Durand D, Gimel J-C (1996) Scattering properties and modelling of aggregating and gelling systems. In: Brown W (ed) Light scattering: principles and development. Clarendon Press, Oxford
Nohara D, Mizutani A, Sakai T (1999) Kinetic study on thermal denaturation of hen egg-white lysozyme involving precipitation. J Biosci Bioeng 87:199–205
Pace CN (1975) The stability of globular proteins. CRC Crit Rev Biochem 3:1–43
Panouille M, Nicolai T, Durand D (2004) Heat induced aggregation and gelation of casein submicelles. Int Dairy J 14:297–303
Pedersen JS (1993) Small-angle scattering from precipitates: analysis by use of a polydisperse hard-sphere model. Phys Rev B 47:657–665
Poon WCK, Egelhaaf SU, Beales PA, Salonen A, Sawyer L (2000) Protein crystallization: scaling of charge and salt concentration in lysozyme solutions. J Phys Condens Matter 12:L569–L574
Povey JF, Smales CM, Hassard SJ, Howard MJ (2007) Comparison of the effects of 2,2,2-trifluoroethanol on peptide and protein structure and function. J Struct Biol 157:329–338
Privalov PL (1979) Stability of proteins: small globular proteins. Adv Protein Chem 33:167–241
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Puertas AM, De Michele C, Sciortino F, Tartaglia P, Zaccarelli E (2007) Viscoelasticity and Stokes–Einstein relation in repulsive and attractive colloidal glasses. J Chem Phys 127:144906
Rosch TW, Errington JR (2007) Investigation of the phase behavior of an embedded charge protein model through molecular simulation. J Phys Chem B 111:12591–12598
Rouse PEJ (1953) A theory of the linear viscoelastic properties of dilute solutions of coiling polymers. J Chem Phys 21:1272–1280
Rueb CJ, Zukoski CF (1998) Rheology of suspensions of weakly attractive particles: approach to gelation. J Rheol 42:1451–1476
Sabulal B, Kishore N (1995) Differential scanning calorimetric study of the interactions of some stabilizing amino acids and oligopeptides with hen egg white lysozyme. J Chem Soc Faraday Trans 91(14):2101–2106
Salvetti G, Tombari E, Mikheeva L, Johari GP (2002) The endothermic effects during denaturation of lysozyme by temperature modulated calorimetry and an intermediate reaction equilibrium. J Phys Chem B 106:6081–6087
Tanford C, Epstein J (1954) The physical chemistry of insulin. I. Hydrogen ion titration curve of zinc-free insulin. J Am Chem Soc 76:2163–2169
ten Wolde PR, Frenkel D (1997) Enhancement of protein crystal nucleation by critical density fluctuations. Science 277:1975–1978
van der Plancken I, Van Loey A, Hendrickx ME (2006) Effect of heat-treatment on the physico-chemical properties of egg white proteins: a kinetic study. J Food Eng 75:316–326
Vetri V, Librizzi F, Leone M, Militello V (2007) Thermal aggregation of bovine serum albumin at different pH: comparison with human serum albumin. Eur Biophys J 36:717–725
Winter HH, Chambon F (1986) Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol 30:367–382
Yan H, Saiani A, Gough JE, Miller AF (2006) Thermoreversible protein hydrogel as cell scaffold. Biomacromolecules 7:2776–2782
Zhang S (2003) Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol 21:1171–1178
Acknowledgments
We thank R. Carrotta, G. Bellavia, A. Cupane, A. Emanuele, M. Leone, R. Noto and V. Vetri for relevant discussions and collaborations. We thank V. Foderà for the help in the use of the AFM instruments. This work was partially supported by the Italian National Research Council through the project “Intermolecular interaction in protein metastable solution”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of the XIX Congress of the Italian Society of Pure and Applied Biophysics (SIBPA), Rome, September 2008.
Rights and permissions
About this article
Cite this article
Raccosta, S., Manno, M., Bulone, D. et al. Irreversible gelation of thermally unfolded proteins: structural and mechanical properties of lysozyme aggregates. Eur Biophys J 39, 1007–1017 (2010). https://doi.org/10.1007/s00249-009-0503-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-009-0503-4