Abstract
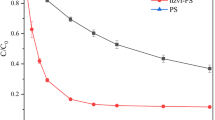
Sulfaquinoxaline (SQX) is a coccidiostatic drug widely used in poultry and swine production and has been frequently detected in various environmental compartments such as surface water, groundwater, soils, and sediments. In the present study, degradation of SQX by ferrous ion-activated peroxymonosulfate oxidation process (Fe(II)/PMS), a promising in situ chemical oxidation (ISCO) technique, was systematically investigated. Experimental results showed that Fe(II)/PMS process appeared to be more efficient for SQX removal relative to Fe(II)/persulfate process (Fe(II)/PS). An optimal Fe(II):PMS molar ratio of 1:1 was found to be necessary for efficient removal of SQX. Increasing the solution pH hampered the degradation of SQX, and no enhancement in SQX degradation was observed when chelating agents S,S′-ethylenediamine-N,N′-disuccinic acid (EDDS) and citrate were present. The presence of Suwannee River fulvic acid (SRFA), as a representative of aquatic natural organic matter (NOM), could inhibit the degradation of SQX. SQX was more susceptible to Fe(II)/PMS oxidation in comparison to its substructural analog 2-amino-quinoxaline (2-AQ) and other sulfonamides, i.e., sulfapyridine (SPD) and sulfadiazine (SDZ). Transformation products of SQX were enriched by solid-phase extraction (SPE) and identified by liquid chromatography-electrospray ionization-triple quadrupole mass spectrometry (LC-ESI-MS/MS). On the basis of the TPs identified, detailed reaction pathways for SQX degradation including sulfonamide bond cleavage, SO2 extrusion, and aniline moiety oxidation were proposed. Our contribution may provide some useful information for better understanding the kinetics and mechanisms of SQX degradation by sulfate radical-based advanced oxidation processes (SR-AOPs).







Similar content being viewed by others
References
Anipsitakis GP, Dionysiou DD (2003) Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ Sci Technol 37:4790–4797
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Antoniou MG, de la Cruz AA, Dionysiou DD (2010) Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl Catal B Environ 96:290–298
Ayoub G, Ghauch A (2014) Assessment of bimetallic and trimetallic iron-based systems for persulfate activation: application to sulfamethoxazole degradation. Chem Eng J 256:280–292
Baran W, Adame E, Ziemiańska J, Sobczak A (2011) Effects of the presence of sulfonamides in the environment and their influence on human health. J Hazard Mater 196:1–15
Bialk-Bielinska A, Stolte S, Arning J, Uebers U, Boschen A, Stepnowski P, Matzke M (2011) Ecotoxicity evaluation of selected sulfonamides. Chemosphere 85:928–933
Boxall A, Kolpin D, Holling-Sorensen B, Tolls J (2003) Are veterinary medicines causing environmental risks? Environ Sci Technol 37:287A–294A
Campbell WC (2008) History of the discovery of sulfaquinoxaline as a coccidiostat. J Parasitol 94(4):934–945
Celiz MD, Tso J, Aga DS (2009) Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ Toxicol Chem 28:2473–2484
Chen Y, Zhang K, Zuo Y (2013) Direct and indirect photodegradation of estriol in the presence of humic acid, nitrate and iron complexes in water solutions. Sci Total Environ 463–464:802–809
Chen K, Zhou JL (2014) Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 95:604–612
David Gara PM, Bosio GN, Gonzalez MC, Russo N, del Carmen MM, Pis Diez R, Mártire DO (2009) A combined theoretical and experimental study on the oxidation of fulvic acid by the sulfate radical anion. Photochem Photobiol Sci 8:992–997
Devi P, Das U, Dalai AK (2016) In-situ chemical oxidation: principle and applications of peroxide and persulfate treatments in wastewater system. Sci Total Environ 571:643–657
Dodd M, Kohler HPE, von Gunten U (2009) Oxidation of antibacterial compounds by ozone and hydroxyl radical: elimination of biological activity during aqueous ozonation processes. Environ Sci Technol 43:2498–2504
Doretto KM, Peruchi LM, Rath S (2014) Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci Total Environ 476–477:406–414
Fan Y, Ji Y, Kong D, Lu J, Zhou Q (2015) Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J Hazard Mater 300:39–47
Furman O, Teel AL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428
García-Galán MJ, Silvia Díaz-Cruz M, Barceló D (2008) Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trends Anal Chem 27:1008–1022
García-Galán MJ, Silvia Díaz-Cruz M, Barceló D (2011) Occurrence of sulfonamide residues along the Ebro river basin removal in wastewater treatment plants and environmental impact assessment. Environ Int 37:462–473
Ghauch A, Ayoub G, Naim S (2013) Degradation of sulfamethoxazole by persulfate assisted micrometric Fe0 in aqueous solution. Chem Eng J 228:1168–1181
Ghauch A, Baalbaki A, Amasha M, Asmar RE, Tantawi O (2017) Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem Eng J 317:1012–1025
Ghauch A, Tuqan AM, Kibbi N (2012a) Ibuprofen removal by heated persulfate in aqueous solution: a kinetic study. Chem Eng J 197:483–492
Ghauch A, Tuqan AM, Kibbi N, Geryes S (2012b) Methylene blue discoloration by heated persulfate in aqueous solution. Chem Eng J 213:259–271
Goldstone JV, Pullin MJ, Bertilsson S, Voelker BM (2002) Reactions of hydroxyl radical with humic substances: bleaching, mineralization, and production of bioavailable carbon substrate. Environ Sci Technol 36:364–372
Hoff RB, Barreto F, Melo J, Jank L, Peralba MDCR, Pizaolato TM (2012) Characterization and estimation of sulfaquinoxaline metabolites in animal tissues using liquid chromatography coupled to tandem mass spectrometry. Anal Methods 4:2822–2830
Hoff RB, Meneghini L, Pizaolato TM, Peralba MDCR, Silvia Díaz-Cruz M, Barceló D (2014) Structural elucidation of sulfaquinoxaline metabolism products and their occurrence in biological samples using high-resolution orbitrap mass spectrometry. Anal Chem 86:5579–5586
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices—a review. J Environ Manag 92:2304–2347
Ikehata K, Jodeiri Naghashkar N, Gamal El-Din M (2006) Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone Sci Eng 28:353–414
Ji Y, Fan Y, Liu K, Kong D, Lu J (2015) Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res 87:1–9
Ji Y, Ferronato C, Salvador A, Yang X, Chovelon JM (2014) Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Environ 472:800–808
Ji Y, Kong D, Lu J, Jin H, Kang F, Yin X (2016) Cobalt catalyzed peroxymonosulfate oxidation of tetrabromobisphenol a: kinetics, reaction pathways, and formation of brominated by-products. J Hazard Mater 313:229–237
Ji Y, Shi Y, Wang L, Lu J, Ferronato C, Chovelon JM (2017) Sulfate radical-based oxidation of antibiotics sulfamethazine, sulfapyridine, sulfadiazine, sulfadimethoxine, and sulfachloropyridazine: formation of SO2 extrusion products and effects of natural organic matter. Sci Total Environ 593–594:704–712
Jiang C, Ji Y, Shi Y, Chen J, Cai T (2016) Sulfate radical-based oxidation of fluoroquinolone antibiotics: kinetics, mechanisms and effects of natural water matrices. Water Res 106:507–517
Keen OS, Linden KG (2013) Degradation of antibiotic activity during UV/H2O2 advanced oxidation and photolysis in wastewater effluent. Environ Sci Technol 47:13020–13030
Kümmerer K (2009a) Antibiotics in the aquatic environment—a review—part I. Chemosphere 75:417–434
Kümmerer K (2009b) Antibiotics in the aquatic environment—a review—part II. Chemosphere 75:435–441
Le Fur C, Legeret B, de Sainte CP, Wong-Wah-Chung P, Sarakha M (2013) Liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry for the analysis of sulfaquinoxaline byproducts formed in water upon solar light irradiation. Rapid Commun Mass Spectrom 27:722–730
Liang C, Bruell CJ, Marley MC, Sperry KL (2004) Persulfate oxidation for in situ remediation of TCE. II. Activated by chelated ferrous ion. Chemosphere 55:1225–1233
Liang C, Huang CF, Chen YJ (2008) Potential for activated persulfate degradation of BTEX contamination. Water Res 42:4091–4100
Liang C, Liang CP, Chen CC (2009) pH dependence of persulfate activation by EDTA/Fe(III) for degradation of trichloroethylene. J Contam Hydrol 106:173–182
Liao QN, Ji F, Li JC, Zhan X, Hu ZH (2016) Decomposition and mineralization of sulfaquinoxaline sodium during UV/H2O2 oxidation processes. Chem Eng J 284:494–502
Liu CS, Shih K, Sun CX, Wang F (2012) Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci Total Environ 416:507–512
Lutze HV, Bircher S, Rapp I, Kerlin N, Bakkour R, Geisler M, von Sonntag C, Schmidt TC (2015) Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ Sci Technol 49:1673–1680
Matzek LW, Carter KE (2016) Activated persulfate for organic chemical degradation : a review. Chemosphere 151:178–188
Naim S, Ghauch A (2016) Ranitidine abatement in chemically activated persulfate systems: assessment of industrial iron waste for sustainable applications. Chem Eng J 288:276–288
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17(3):1027–1284
Neta P, Madhavan V, Zemel H, Fessenden RW (1977) Rate constants and mechanism of reaction of SO4 •- with aromatic compounds. J Am Chem Soc 99:163–164
Nfodzo P, Choi H (2011) Triclosan decomposition by sulfate radicals: effects of oxidant and metal doses. Chem Eng J 174:629–634
Nie M, Yan C, Li M, Wang X, Bi W, Dong W (2015) Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem Eng J 279:507–515
Pignatello JJ, Oliveros E, Mackay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36(1):1–84
Qi C, Liu X, Lin C, Zhang X, Ma J, Tan H, Ye W (2014) Degradation of sulfamethoxazole by microwave-activated persulfate: kinetics, mechanism and acute toxicity. Chem Eng J 249:6–14
Qi C, Liu X, Ma J, Lin C, Li X, Zhang H (2016) Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants. Chemosphere 213:259–271
Rao YF, Qu L, Yang H, Chu W (2014) Degradation of carbamazepine by Fe(II)-activated persulfate process. J Hazard Mater 268:23–32
Rastogi A, Al-Abed SR, Dionysiou DD (2009a) Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res 43:684–694
Rastogi A, Al-Abed SR, Dionysiou DD (2009b) Sulfate radical-based ferrous-peroxymonosulfate oxidative system for degradation in aqueous and sediment systems. Appl Catal B Environ 85:171–179
Rodriguez S, Vasquez L, Costa D, Romero A, Santos A (2014) Oxidation of orange G by persulfate activated by Fe(II), Fe(III) and zero valent iron (ZVI). Chemosphere 101:86–92
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry. Wiley, Hoboken ISBN 0-471-35750-2
Stumm W, Fred Lee G (1961) Oxygenation of ferrous ion. Ind Eng Chem 53:143–146
Tentscher PR, Eustis SN, McNeill K, Samuel Arey J (2013) Aqueous oxidation of sulfonamide antibiotics: aromatic nucleophilic substitution of an aniline radical cation. Chem Eur J 19:11216–11223
Tistonaki A, Petri B, Crimi M, Mosbæk H, Siegrist RL, Bjerg PL (2010) In situ chemical oxidation of contaminated soil and groudwater using persulfate: a review. Crit Rev Environ Sci Technol 40:55–91
Vione D, Falletti G, Maurino V, Minero C, Pelizzetti E, Malandrino M, Ajassa R, Olariu RI, Arsene C (2006) Sources and sinks of hydroxyl radicals upon irradiation of natural water samples. Environ Sci Technol 40:3775–3781
Wang YR, Chu W (2011) Degradation of a xanthene dye by Fe(II)-mediated activation of Oxone process. J Hazard Mater 186:1445–1461
Wei R, Ge F, Huang S, Chen M, Wang R (2011) Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu province, China. Chemosphere 82:1408–1414
Wenk J, Canonica S (2012) Phenolic antioxidants inhibit the triplet-induced transformation of anilines and sulfonamide antibiotics in aqueous solution. Environ Sci Technol 46:5455–5462
Wenk J, von Gunten U, Canonica S (2011) Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical. Environ Sci Technol 45:1334–1340
Westerhoff P, Mezyk SP, Cooper WJ, Minakata D (2007) Electron pulse radiolysis determination of hydroxyl radical rate constants with Suwannee river fulvic acid and other dissolved organic matter isolates. Environ Sci Technol 41(13):4640–4646
Yan C, Yang Y, Zhou J, Liu M, Nie M, Shi H, Gu L (2013) Antibiotics in the surface water of the Yangtze estuary: occurrence, distribution and risk assessment. Environ Pollut 175:22–29
Zhao L, Ji Y, Kong D, Lu J, Zhou Q, Yin X (2016) Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem Eng J 303:458–466
Zhou L, Ferronato C, Chovelon JM, Sleiman M, Richard C (2017) Investigations of diatrizoate degradation by photo-activated persulfate. Chem Eng J 311:28–36
Zhou L, Zheng W, Ji Y, Zhang J, Cao Z, Zhang Y, Wang Q, Yang X (2013) Ferrous-activated persulfate oxidation of arsenic (III) and diuron in aquatic system. J Hazard Mater 263:422–430
Zuo Y (ed) (2014) High-performance liquid chromatography (HPLC): principles, procedures and practices. Nova Science Publishers, Inc., New York
Zuo Y, Hoigné J (1992) Formation of H2O2 and decomposition of oxalate in atmospheric water by sunlight inducing photolysis of Fe(III) oxalate complexes. Environ Sci Technol 26:1014–1022
Zuo Y, Jones R (1997) Photochemistry of natural dissolved organic matter in lake and wetland waters-production of carbon monoxide. Water Res 31:850–858
Zuo Y, Zhan J (2005) Effects of oxalate on Fe-catalyzed photooxidation of dissolved sulfur dioxide in atmospheric water. Atmos Environ 39:27–37
Acknowledgements
The authors gratefully acknowledge the financial support from the Natural Science Foundation of Jiangsu Province-China (Grant No. BK20160709), the National Natural Science Foundation of China (Grant No. 21607077), the Fundamental Research Funds for Central Universities (Grant No. KJQN201741), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institute. The content of the paper does not necessarily represent the views of the funding agencies. We gratefully acknowledge two anonymous reviewers for their valuable comments and constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Rights and permissions
About this article
Cite this article
Ji, Y., Wang, L., Jiang, M. et al. Ferrous-activated peroxymonosulfate oxidation of antimicrobial agent sulfaquinoxaline and structurally related compounds in aqueous solution: kinetics, products, and transformation pathways. Environ Sci Pollut Res 24, 19535–19545 (2017). https://doi.org/10.1007/s11356-017-9569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9569-1