Abstract
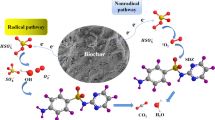
In this paper, three types of black carbons (BCs) named R-BC, W-BC, and C-BC were derived from rice straw ashes, wheat straw ashes, and corn straw ashes, respectively. Under room temperature and in an anaerobic aqueous solution, these three types of BCs could catalyze the reduction of nitrobenzene (NB) by sulfides rather than only act as the superabsorbent. The catalytic activities of BCs derived from different crop-residue ashes were very different and in the order of R-BC > W-BC > C-BC, since the reaction rate constants (k obs) of NB with the BCs in the presence of 3 mM sulfides were 0.0186, 0.0063, and 0.0051 h−1, respectively. The key catalytic active sites for NB reduction were evaluated, with four types of modified BCs and two types of tailored graphite as the model catalysts. The results indicated that BCs probably had two types of active sites for NB reduction, the microscopic graphene moieties and the surface oxygen functional groups. Since the sulfides and BCs often coexist in the environment, this BC-catalyzed reduction technology of NACs may be applied as an in situ remediation technique without the need for reagent addition.






Similar content being viewed by others
References
Amezquita-Garcia HJ, Razo-Flores E, Cervantes FJ, Rangel-Mendez JR (2013) Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon 55:276–284
Boehm HP (1996) Chemical identification of surface groups. Adv Catal 16:179–274
Brändli RC, Hartnik T, Henriksen T, Cornelissen G (2008) Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soil. Chemosphere 73:1805–1810
Chen BL, Huang WH (2011) Effects of compositional heterogeneity and nanoporosity of raw and treated biomass-generated soot on adsorption and absorption of organic contaminants. Environ Pollut 159:550–556
Chen P, McCreery RL (1996) Control of electron transfer kinetics at glassy carbon electrodes by specific surface modification. Anal Chem 68:3958–3965
Chen M, Cui L, Li CH, Diao GW (2009) Adsorption, desorption and condensation of nitrobenzene solution from active carbon: a comparison of two cyclodextrins and two surfactants. J Hazard Mater 162:23–28
Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, Noort PMV (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895
Cui XY, Wang HL, Lou LP, Chen YX, Yu YL, Shi JY, Xu L, Khan MI (2009) Sorption and genotoxicity of sediment-associated pentachlorophenol and pyrene influenced by crop residue ash. J Soil Sediment 9:604–612
Dunnivant FM, Schwarzenbach RP, Macalady DL (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic-matter. Environ Sci Technol 26:2133–2141
Forbes MS, Raison RJ, Skjemstad JO (2006) Formation, transformation, and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci Total Environ 370:190–206
Huang JH, Hsu SH, Wang SL (2011) Effects of rice straw ash amendment on Cu solubility and distribution in flooded rice paddy soils. J Hazard Mater 186:1801–1807
Hummers WS, Offeman RE (1958) Preparation of graphite oxide. J Am Chem Soc 180:1339
Kamegawa K, Nishikubo K, Yoshida H (1998) Oxidative degradation of carbon blacks with nitric acid (I)—changes in pore and crystallographic structures. Carbon 36:433–441
Kemper JM, Ammar E, Mitch WA (2008) Abiotic degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine in the presence of hydrogen sulfide and black carbon. Environ Sci Technol 42:2118–2123
Langley LA, Fairbrother DH (2007) Effect of wet chemical treatments on the distribution of surface oxides on carbonaceous materials. Carbon 45:47–54
Larsen JW, Freund M, Kim KY, Sidovar M, Stuart JL (2000) Mechanism of the carbon catalyzed reduction of nitrobenzene by hydrazine. Carbon 38:655–661
Lee DW, Seo JW (2011) Formation of phenol groups in hydrated graphite oxide. J Phys Chem C 115:12483–12486
Masiello CA, Druffel ERM (1998) Black carbon in deep-sea sediments. Science 280:1911–1913
Min G, Wang S, Zhu HP, Fang GZ, Zhang Y (2008) Multi-walled carbon nanotubes as solid-phase extraction adsorbents for determination of atrazine and its principal metabolites in water and soil samples by gas chromatography-mass spectrometry. Sci Total Environ 396:79–85
Oh SY, Chiu PC (2009) Graphite- and soot-mediated reduction of 2,4-dinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine. Environ Sci Technol 43:6983–6988
Oh SY, Cha DK, Chiu PC (2002) Graphite-mediated reduction of 2,4-dinitrotoluene with elemental iron. Environ Sci Technol 36:2178–2184
Oh SY, Cha DK, Chiu PC (2004) Reduction of nitroglycerin with cast iron: pathway, kinetics, and mechanisms. Environ Sci Technol 38:3723–3730
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. John Wiley & Sons, New Jersey
Sheng GY, Yang YN, Huang MS, Yang K (2005) Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ Pollut 134:457–463
Stankovich S, Piner RD, Nguyen ST, Ruoff RS (2006) Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 44:3342–3347
Streets DG, Yarber KF, Woo JH, Carmichael GR (2003) Biomass burning in Asia: annual and seasonal estimates and atmospheric emissions. Glob Biogeochem Cy 17:1099–1118
Sun K, Zhang ZY, Gao B, Wang ZY, Xu DY, Jin J, Liu XT (2012) Adsorption of diuron, fluridone and norflurazon on single-walled and multi-walled carbon nanotubes. Sci Total Environ 439:1–7
Teng HS, Tu YT, Lai YC, Lin CC (2001) Reduction of NO with NH3 over carbon catalysts―the effects of treating carbon with H2SO4 and HNO3. Carbon 39:575–582
Valdes H, Sanchez-Polo M, Rivera-Utrilla J, Zaror CA (2002) Effect of ozone treatment on surface properties of activated carbon. Langmuir 18:2111–2116
Van der Zee FP, Bisschops IAE, Lettinga G (2003) Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environ Sci Technol 37:402–408
Xu W, Dana KE, Mitch WA (2010) Black carbon-mediated destruction of nitroglycerin and RDX by hydrogen sulfide. Environ Sci Technol 44:6409–6415
Yang Y, Sheng GY (2003) Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ Sci Technol 37:3635–3639
Yu XD, Gong WW, Liu XH, Shi L, Han X, Bao HY (2011) The use of carbon black to catalyze the reduction of nitrobenzenes by sulfides. J Hazard Mater 198:340–346
Yu XD, Gong WW, Liu XH, Bao HY (2012) The reductive mechanism of nitrobenzene catalyzed by nine charcoals in sulfides solution. Sci China Chem 55:1–7
Zeng XY, Ma YT, Ma LR (2007) Utilization of straw in biomass energy in China. Renew Sust Energ Rev 11:976–987
Zhong HP, Yue YZ, Fan JW (2003) Chracterization of crop straw resources in China and its utilization. Resour Sci 25:62–67
Zhou HY, Shi L, Sun Q (2012) Reduction of nitrobenzene with hydrazine hydrate catalyzed by CCID-treated activated carbon. Chin J Catal 33:1463–1469
Zhu D, Pignatello JJ (2005) Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ Sci Technol 39:2033–2041
Acknowledgments
The research was financially supported by the National Science Foundation for Innovative Research Group (51121003), Major State Basic Research Development Program (2013CB430405), National Natural Science Foundation of China (20977009), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Gong, W., Liu, X., Tao, L. et al. Reduction of nitrobenzene with sulfides catalyzed by the black carbons from crop-residue ashes. Environ Sci Pollut Res 21, 6162–6169 (2014). https://doi.org/10.1007/s11356-014-2533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2533-4