Abstract
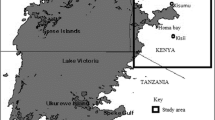
The formation of perfluorooctanoate (PFOA) from 1H,1H,2H,2H-perfluorodecanol (8:2 FTOH) was studied for the first time in laboratory experiments with brackish water. The water samples were collected from the Baltic Sea, which is one of the largest brackish water areas in the world and is polluted with PFOA and other perfluorinated compounds. The formation of PFOA was studied in closed-bottle experiments at different water temperatures. As a reference experiment, a modified OECD 310 test was conducted with sludge from a wastewater treatment plant and with brackish water. The PFOA and 8:2 FTOH were concentrated from water samples by solid-phase extraction (SPE) and were analysed using liquid chromatography–mass spectrometry. The effect of oxygen concentration on the formation of PFOA was studied using surface water samples with high and low oxygen contents. Other experiments were performed with oxygen-rich surface water and oxygen-deficient bottom water. The formation of PFOA was observed in all experiments; it was higher in the trial performed with brackish water than in the reference test carried out with sludge. Clear temperature dependence was observed in the formation of PFOA in brackish water tests; after a 30-day test period, a sixfold increase was observed in the amount of PFOA in surface water between the temperatures of 15 and 20 °C. Microbes were suggested as the major cause of the formation of PFOA, but other environmental characteristics, such as oxygen, could also affect the formation potential of PFOA.





Similar content being viewed by others
References
Ahrens L, Shoeib M, Harner T, Lee SC, Guo R, Reiner EJ (2011) Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere. Environ Sci Technol 45:8098–8105
Arvaniti OS, Ventouri EI, Stasinakis AS, Thomaidis NS (2012) Occurrence of different classes of perfluorinated compounds in Greek wastewater treatment plants and determination of their solid–water distribution coefficients. J Hazard Mater 239–240:24–31
Becker AM, Gerstmann S, Frank H (2008) Perfluorooctane surfactants in waste waters, the major source of river pollution. Chemosphere 72:115–121
Celander MC (2011) Cocktail effects on biomarker responses in fish. Aquat Toxicol 105:72–77
Dinglasan MJ, Ye Y, Edwards EA, Mabury SA (2004) Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol 38:2857–2864
Ellington JJ, Washington JW, Evans JJ, Jenkins TM, Hafner SC, Neill MP (2009) Analysis of fluorotelomer alcohols in soils: optimization of extraction and chromatography. J Chromatogr A 1216:5347–5354
Fiedler S, Pfister G, Schramm K-W (2010) Poly- and perfluorinated compounds in household consumer products. Toxicol Environ Chem 92:1801–1811
Gauthier SA, Mabury SA (2005) Aqueous photolysis of 8:2 fluorotelomer alcohol. Environ Toxicol Chem 24:1837–1846
Hoikkala L, Lahtinen T, Perttilä M, Lingell R (2012) Seasonal dynamics of dissolved organic matter on a coastal salinity gradient in the northern Baltic Sea. Cont Shelf Res 45:1–14
Kaartokallio H, Tuomainen J, Kuosa H, Kuparinen J, Martikainen PJ, Servomaa K (2008) Succession of sea-ice bacterial communities in the Baltic Sea fast ice. Polar Biol 31:783–793
Kallenborn R, Berger U, Järnberg U (2004) Perfluorinated alkylated substances (PFAS) in the Nordic environment. Nordic Council of Ministers, Copenhagen, p 552
Kissa E (2001) Fluorinated surfactants and repellents, 2nd edn. Marcel Dekker, New York
Larsen BS, Stchur P, Szostek B, Bachmura SF, Rowand RC, Prickett KB, Korzeniowski SH, Buck RC (2006) Method development for the determination of residual fluorotelomer raw materials and perflurooctanoate in fluorotelomer-based products by gas chromatography and liquid chromatography mass spectrometry. J Chromatogr A 1110:117–124
Liu J, Lee LS, Nies LF, Nakatsu CH, Turco RF (2007) Biotransformation of 8:2 fluorotelomer alcohol in soil and by soil bacteria isolates. Environ Sci Technol 41:8024–8030
Loganathan BG, Sajwan KS, Sinclair E, Senthil Kumar K, Kannan K (2007) Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res 41:4611–4620
Mahmoud MAM, Kärrman A, Oono S, Harada KH, Koizumi A (2009) Polyfluorinated telomers in precipitation and surface water in an urban area of Japan. Chemosphere 74:467–472
Martin JW, Kannan K, Berger U, De Voogt P, Field J, Franklin J, Giesy JP, Harner T, Muir DCG, Scott B, Kaiser M, Järnberg U, Jones KC, Mabury SA, Schroeder H, Simcik M, Sottani C, Van Bavel B, Kärrman A, Lindström G, Van Leeuwen S (2004) Analytical challenges hamper perfluoroalkyl research. Environ Sci Technol 38:248A–255A
OECD (2006) Guidelines for the Testing of Chemicals Test No. 310: Ready Biodegradability—CO2 in sealed vessels (Headspace Test). OECD, Paris
Phillips MM, Dinglasan-Panlilio MJA, Mabury SA, Solomon KR, Sibley PK (2007) Fluorotelomer acids are more toxic than perfluorinated acids. Environ Sci Technol 41:7159–7163
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and fransport of perfluorocarboxylates. Environ Sci Technol 40:32–44
Russell MH, Berti WR, Szostek B, Buck RC (2008) Investigation of the biodegradation potential of a fluoroacrylate polymer product in aerobic soils. Environ Sci Technol 42:800–807
Sáez M, de Voogt P, Parsons JR (2008) Persistence of perfluoroalkylated substances in closed bottle tests with municipal sewage sludge. Environ Sci Pollut Res 15:472–477
Stock NL, Lau FK, Ellis DA, Martin JW, Muir DCG, Mabury SA (2004) Polyfluorinated telomer alcohols and sulfonamides in the North American troposphere. Environ Sci Technol 38:991–996
Szostek B, Prickett KB, Buck RC (2006) Determination of fluorotelomer alcohols by liquid chromatography/tandem mass spectrometry in water. Rapid Commun Mass Spectrom 20:2837–2844
Taniyasu S, Kannan K, So MK, Gulkowska A, Sinclair E, Okazawa T, Yamashita N (2005) Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A 1093:89–97
van Zelm R, Huijbregts MAJ, Russell MH, Jager T, van de Meent D (2008) Modeling the environmental fate of perfluorooctanoate and its precursors from global fluorotelomer acrylate polymer use. Environ Toxicol Chem 27:2216–2223
Vierke L, Staude C, Biegel-Engler A, Drost W, Schulte C (2012) Perfluorooctanoic acid (PFOA)- main concerns and regulatory developments in Europe from an environmental point of view. Environ Sci Eur 24:16
Wang N, Szostek B, Folsom PW, Sulecki LM, Capka V, Buck RC, Berti WR, Gannon JT (2005) Aerobic biotransformation of 14C-labeled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ Sci Technol 39:531–538
Wang N, Szostek B, Buck RC, Folsom PW, Sulecki LM, Gannon JT (2009) 8-2 fluorotelomer alcohol aerobic soil biodegradation: pathways, metabolites, and metabolic yields. Chemosphere 75:1089–1096
Xiao F, Halbach TR, Simcik MF, Gulliver JS (2012) Input characterization of perfluoroalkyl substances in wastewater treatment plants: Source discrimination by exploratory data analysis. Water Res 46:3101–3109
Yoo H, Washington JW, Ellington JJ, Jenkins TM, Neill MP (2010) Concentrations, distribution, and peristence of fluorotelomer alcohols in sludge-applied soils near Decatur, Alabama, USA. Environ Sci Technol 44:8397–8402
Acknowledgment
We gratefully acknowledge the financial support of the Maj and Tor Nessling Foundation through a grant awarded for this research. We thank the laboratory staff of Tvärminne Zoological Station for assistance in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Keränen, J., Ahkola, H., Knuutinen, J. et al. Formation of PFOA from 8:2 FTOH in closed-bottle experiments with brackish water. Environ Sci Pollut Res 20, 8001–8012 (2013). https://doi.org/10.1007/s11356-013-1975-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1975-4