Abstract
The Great Zab River catchment is a major left-bank tributary of the River Tigris and drains a substantial part of the Kurdistan Region, an autonomous region of Northern Iraq. Within Kurdistan, the water resources of the Great Zab River catchment are under pressure from population increase and are utilized for potable, domestic and agricultural and industrial supply. As with many parts of the world, effective management of water resources within Kurdistan is hindered by a lack of water quality data and established background concentrations. This study therefore represents the first regional survey of river water chemistry for the Great Zab River catchment and presents data on the spatial and temporal trends in concentrations of As, Ba, Ca, Cd, Co, Cr, Cu, Fe, Hg, Li, Mn, Mo, Ni, Pb, Sr, Zn, NO3−, SO42−, F−, Cl− and PO43−, in addition to pH, electrical conductivity, dissolved oxygen and turbidity. As a tool for underpinning the management and monitoring of water quality, background concentrations were defined for the Great Zab catchment using three methods. The influences of geogenic and anthropogenic controls upon spatial and temporal trends in water chemistry are also evaluated. The influence of geogenic loading from underlying bedrock was identifiable within the observed spatial trends, with the most notable differences found between waters sampled from the relatively more volcanic-rich Zagros zone to the north and those sampled from the lower catchment underlain by younger clay-, sand- and siltstones. The greatest anthropogenic influence, identifiable through elements such as Cl− and NO3−, is present in the more highly populated lower catchment. The background concentrations identified in the Great Zab catchment would be those expected as a result of geogenic loading with some anthropogenic influence and represent a more conservative value when compared to those such as the World Health Organization Maximum Admissible Concentration. However, background concentrations represent a powerful tool for identifying potential anthropogenic impacts on water quality and informing management of such occurrences.
Similar content being viewed by others
Introduction
As noted by Adams and Chapman (2007), compared to organic substances, metals in surface waters pose significant regulatory challenges given that they may be sourced from natural geogenic loading as well as anthropogenic inputs, and that the latter may be highly spatially variable and diffuse in nature. Furthermore, anthropogenic inputs may stem from historical activities long-since ceased (e.g. Alpers et al. 2005).
A major step in developing a regulatory framework for surface water quality is the derivation of background concentrations (Oste et al. 2012). Determining a background concentration may be used to set a threshold value, which may allow instances of contamination of water to be identified (Panno et al. 2006). Background can be considered to be a concentration of a substance resulting from natural processes, uninfluenced by human activity (Reimann and Garrett 2005). Given the spatially variability in factors determining geogenic metal loading to surface waters, ‘global’ background values are therefore of limited use (Oste et al. 2012). As a result, developing regional background concentrations can be seen as preferable. Given the difficulty in finding waters that have no anthropogenic ‘signal’ (Smith et al. 2003), the term ‘ambient background concentrations’ has been developed, whereby concentrations represent natural and low level anthropogenic inputs (Peters et al. 2012). Approaches to defining background concentrations in surface waters have been reviewed by detail by Oste et al. (2012).
Ensuring water quality is a key component of ensuring sufficient water supply as part of water resources management. This is particularly relevant to the provision of potable water supplies. Pressures on water quality have been noted in catchments and regions experiencing population and industrial growth (McDonald et al. 2011, 2014).
The Kurdistan region is experiencing rapid increase in population. This combined with an increased urban population, and improvements in living standards have resulted in pressures on water resources through impacts upon water quality (Alobaidy et al. 2010). These pressures are being felt, in conjunction with the threat posed from climate change, across the globe (Vörösmarty et al. 2000). The Great Zab River catchment represents one of the largest catchments in the Kurdistan Region, covering an area of approximately 40,643 km2, and surface waters in the catchment provide water supply to a population of 1.8 million. Effective management of water resources through legislative control on water quality is currently being restricted by a lack of national water quality guidelines (Shareef and Muhamad 2008). Whilst there has been some study of water quality in particular parts of the catchment (e.g. Abdulwahid 2013; Shareef et al. 2009; Shareef and Muhamad 2008; Toma 2011a, b), there has been no attempt to establish baseline water chemistry and define background concentrations that may act as a means of evaluating water quality, and which may form the basis for the development of appropriate legislative guidelines.
The aims of this study are therefore to address this knowledge gap by determining ambient background concentrations for the Great Zab River catchment for 16 metals, 5 anions, pH, electrical conductivity (EC), dissolved oxygen (DO) and turbidity and to evaluate the spatial and seasonal trends in water quality within the catchment.
Study area
Kurdistan occupies an area of 40,643 km2, and a population growth rate is 2.7% per annum (Ministry of Planning 2011), notably in the cities of Erbil, the capital city, Sulaimaniya and Dohuk, is placing pressure upon water resources. Stevanovic and Iurkiewicz (2009) report that that population growth has led to the decline in the per capita availability and use of water resources. According to the Ministry of Planning/KRG (2011), the quantity of water produced on a daily basis is 924,600 m3, whilst daily demand totals approximately 1.25 million m3. Therefore, the present water supply deficit is 325,400 m3 per day, i.e. 26% of total demand and 35% of total quantity produced at present.
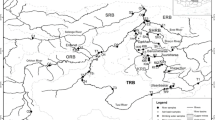
The Great Zab River catchment covers an area of approximately 40,300 km2, with 13,708 km2 within Kurdistan and the remainder in Turkey. The Great Zab River is a left-bank tributary of the River Tigris, with confluence downstream of Mosul. The flow regime of the Great Zab River is strongly influenced by annual snowmelt, with the highest monthly mean discharges occurring in the early spring months (Saleh 2010). Within Kurdistan, the Great Zab catchment sits within three major geological zones (Fig. 1). To the northeast, the Zagros zone comprises predominantly folded limestones with some felsitic volcanics. The imbricated and high-fold zones comprise Cretaceous-age carbonates in a range of different formations. A detailed description of these has been produced by Sissakian (2013); however, in summary these formations comprise predominantly limestone that has been variably pervaded by dolomite, marl and shale. The lower portion of the catchment lies within the foothill zone (Fig. 1), dominated by late Miocene to Pleistocene-aged sandstones, siltstone and claystone (Sissakian 2013). The region at the boundary of the high-fold and foothill zones comprises a number of formations of generally Paleocene and Eocene age that comprise dolomitic limestones and, particularly to the north-west, thick basaltic lavas (Sissakian 2013).
Methods
Samples of river water were collected from 62 sites in February 2014 and June 2014 (Fig. 1). Sample sites were selected to best balance the demands of ease of access and spatial coverage, with sample sites focusing mainly upon road–river intersections (c.f. Bird et al. 2010). In the field, samples were filtered through 0.45-µm cellulose nitrate filter membranes, acidified with 50% HNO3 and placed on ice in acid-washed Nalgene bottles. Samples were stored below 5 °C prior to analysis. Concentrations of As, Ba, Ca, Cd, Co, Cr, Cu, Fe, Hg, Li, Mn, Mo, Ni, Pb, Sr, Zn were determined by inductively coupled plasma mass spectrometry (Agilent Technologies 7700). Analytical accuracy was assessed through the analysis of the NIST1643 certified reference material and analytical precision through the repeat analysis (n = 10% of total sample number) of a randomly selected sample. Data for analytical quality control are given in Table 1. Samples were analysed by ion chromatography for their NO3−, SO42−, F−, Cl− and PO43− content. A Thermo Fisher AS22 anion exchange column was used for the separation of SO42−, F−, Cl− and PO43−. The mobile phase was 4.5 mM sodium carbonate/1.4 mM sodium bicarbonate and flow rate 1.2 ml/min. Suppression was achieved using a Metrohm Suppressor Module, supplied with 150 mM sulphuric acid/0.1 M oxalic acid/acetone mix and ultrapure water. Inorganic anions were detected using a suppressed conductivity detector. All solutions were prepared in ultrapure water with a specific resistance of (18.2) MΩ cm (Millipore, Molsheim, France). Analytical accuracy of anion analyses (Table 1) was assessed through the analysis of standard solutions and analytical precision through the repeat analysis (n = 10% of total sample number) of a randomly selected sample.
In the field, measurements of pH (Hanna Instruments HI991003), electrical conductivity (Hanna Instruments HI8733), dissolved oxygen (PDO520) and turbidity (Hanna Instruments HI93703) were taken at the time of sampling.
Results and discussion
Water chemistry
A summary of minimum, mean, median and maximum values is provided for cations (Fig. 2), anions (Fig. 3) and pH, EC, DO, turbidity (Fig. 4). Global median and World Health Organization (WHO) Maximum Admissible Concentrations (MACs) also plotted in order to provide context for the data from Kurdistan. WHO MACs are appropriate for potable water, which is consistent with the Great Zab River, and provides an indication of water quality in relation to human health. In addition, percentage cumulative frequency plots of the water chemistry data for cations (Fig. 5) and anions, pH, EC, DO, turbidity (Fig. 6) show the frequency distribution of the parameters in summer and winter. Arsenic and Hg were non-detectable in all samples.
In comparison with world median values, cation concentrations in the Great Zab catchment vary by metal (Fig. 2). All Li and Ca concentrations are greater than the world median value, whilst the third quartile concentration for Ba is greater than the world median concentration. Third quartile concentrations for Cu, Zn and Pb are lower than the respective world median concentrations. For metals for which a WHO MAC has been defined, all concentrations in the Great Zab catchment fall below these.
All anion concentrations measured in the Great Zab catchment fall below their respect WHO MACs (Fig. 3). All F− and NO2− concentrations measured in the Great Zab catchment fall below world median concentrations; however, a majority of sites had SO42− concentrations in excess of the world median. The median Cl− concentration in the Great Zab (6.7 mg l−1) is largely similar to the world median concentration of 8.3 mg l−1.
The pH of river water ranges from mildly acidic (5.6) to mildly alkaline (8.9). The turbidity of water (1.7-65 NTU) indicates that in general river water in Great Zab River catchment contains relatively low concentrations of suspended organic and inorganic material. Electrical conductivity ranges from 200 to 1028 µS cm−1 with a median value of 375 µS cm−1.
A Pearson product-moment correlation was performed to evaluate bivariate relationships between water chemistry parameters. The full correlation matrix is included as supplementary material, and Fe, As and Hg were not included in the analysis due to the high number of non-detectable concentrations. Statistically significant correlations between cations and anions are relatively rare, notable exceptions being between SO42− (r = 0.408) and NO3− (r = 0.441) and Ba, both significant at α = 0.01. Statistically significant correlation between cations and between anions is more frequent. With respect to cations, significant correlations exist between metals in the same groups, such as between the transition metals. Particularly strong correlations exist between Ni and Co (r = 0.801), Cu (r = 0.659) and Zn (r = 0.816). For alkaline earth metals, strong correlation relationships exist between Ca and Sr (r = 0.822) and Ba (r = 0.349). Significant correlations exist between a number of anions (see supplementary data), being strongest between F− and SO42− (r = 0.637). There are relatively few significant correlations between elemental concentrations and pH, Ca, Ba, Cl− and NO3− being exceptions. Dissolved oxygen concentrations are correlated with PO43− and SO42− (α = 0.01).
In order to establish whether water chemistry differs significantly between summer (lower flow conditions) and winter (higher flow conditions), data collected in February 2014 and June 2014 were compared using a one-way ANOVA on log-transformed data, using a significance threshold of α = 0.05. Results (Table 2) indicate that concentrations of Ca, Li, Mo are higher in the winter than summer (statistically significant at α = 0.05), whilst concentrations of Zn, SO42−, F−, Cl−, and PO43− are higher in the summer than winter (statistically significant at α = 0.05). Seasonal differences for other analytes were not statistically significant. Higher concentrations of a number of elements during winter (generally higher discharge conditions) in the Great Zab catchment suggest enhanced delivery from the riparian zone via a combination of surface and sub-surface flows. Calcium, Li and SO42− concentrations have been found to be consistently higher than world median concentrations.
The relationship between water quality and river discharge is a potentially complex scenario. Previous studies (e.g. Garbarino et al. 1995) have reported generally higher concentrations of metals under low flow conditions due to a lower degree of physical dilution that occurs during periods of low discharge. In addition, higher discharge conditions can result in a reduction in dissolved concentrations due an increase in sorption process due to higher suspended sediment concentrations. However, conversely other studies (e.g. Gundersen and Steinnes 2001) have demonstrated that periods of higher discharge can yield higher metal concentrations due to enhanced delivery of substances to the river channel from run-off and the potential activation of point sources. What is apparent is that different elements can display different relationships with discharge, as noted in a study of Southeast Asian rivers by Chanpiwat and Sthiannopkao (2014).
In an attempt to evaluate the spatial trends in water quality, samples were grouped based upon the major underlying geological units identified in the Great Zab catchment. The groups were identified as: (1) Zagros zone, (2) the imbricated/high-fold zones and (3) the foothill zone (Fig. 1). The grouping by underlying geology reflects the importance of geogenic loading in determining surface water chemistry (Drever 1997). Log-transformed data were analysed using a one-way ANOVA, using a significance threshold of α = 0.05. The most frequent differences were found between water chemistry in samples collected from the Zagros and foothill zones, with 13 parameters showing a statistically significant difference (Table 2). Five and 7 parameters, respectively, showed statistically significant differences between the Zagros and imbricated/high-fold zones and imbricated/high-fold and foothill zones. The greater statistical similarity of water chemistry sampled in the imbricated/high-fold and foothill zones will reflect the greater similarity in bedrock geology between these areas compared with the Zagros zone. The influence of geology on water chemistry is exemplified by Ca and Sr concentrations; these are found to be significantly higher in waters within the imbricated/high-fold and foothill zones than in the Zagros zones (Table 2). Average Sr concentrations in water with the imbricated/high-fold (542 µg l−1) and foothill zones (683 µg l−1) are at least twice as high as in the Zagros zone (243 µg l−1). Average Ca concentrations are c. 10 mg l−1 lower in the Zagros zone compared to the imbricated/high-fold and foothill zones. This reflects the abundant carbonate bedrocks, notably limestone and dolomitic limestone, which are the main sources of Sr to stream water in particular, whilst the bedrock of Zagros zone is relatively carbonate poor.
Chloride and NO3− show a statistically significant difference between the foothill zone (the lower Great Zab catchment) and the two upstream zones (Table 2). Concentrations of Cl− and NO3−are significantly higher in the lower catchment (Fig. 7), as indicated by mean concentrations of 14.2 and 6.8 mg l−1, respectively, which are twice as high as those for the Zagros and imbricated/high-fold zones upstream. Chloride and NO3− are two substances for which higher concentrations in river water, and particularly within-catchment variability, can be the result of anthropogenic inputs, such as from agricultural activity in the case NO3− (Randall and Mulla 2001). Importantly, the amount of anthropogenic activity is greatest in the lower Great Zab catchment, the part of the catchment containing the highest Cl− and NO3− levels. Whilst concentrations are below respective WHO MACs, the data exemplify and indicate that whilst geogenic inputs are important in explaining large-scale variability in water chemistry, the spatially variable influence of anthropogenic activity is an important contributor to the water chemistry of the Great Zab catchment.
Quantifying background conditions
Establishing background geochemical conditions provides a potentially valuable regulatory tool for evaluating water quality. However, given the difficulty in locating ‘pristine’ water bodies that have no anthropogenic influence, as reviewed by Peters et al. (2012), a range of approaches have been utilized to quantify background conditions in river catchments. Peters et al. (2012) used a low percentile of water quality data to provide a relatively conservative estimate of background, or what can be termed ‘ambient background’. In contrast, other approaches have utilized higher percentile, such as the 90th percentile value (Fraters et al. 2001).
To evaluate the potential influence of the approach used, three different approaches were taken. Firstly, background conditions were calculated as the 10th percentile value (Peters et al. 2012) of the whole data set from the Great Zab River catchment (Table 3). Secondly, based upon the analysis of Q–Q plots of NO3− concentrations, utilized as an anthropogenic marker, and other water quality parameters (Fig. 8), the 95th percentile was identified as a point where the distributions differ (Helsel and Hirsch 2002). Therefore, this is indicative of a different sample population above the 95th percentile and can be interpreted as a separation of geogenic and anthropogenic components (Table 3). Finally, following the method of Davies (1983) background concentrations were estimated using regression analysis of % cumulative frequency curves of log10 concentrations from the sample population (Table 3).
Generally, of the three approaches, the 10th percentile concentrations are always the lowest (Table 3), with the exception of Ca and Sr, for which the regression-derived concentrations are lowest. The 10th percentile concentrations have been described by Peters et al. (2012) as reflecting ambient background conditions that, in relation to the Great Zab catchment, would be expected resulting from natural, geogenic inputs and low level anthropogenic activity. However, utilizing NO3− concentrations as an anthropogenic marker suggests that the proportion of the sample population that could be considered as geogenic is much larger, equating to the 95th percentile value and that background concentrations could potentially be established at higher concentrations.
Given that background concentrations may be used as an indicator of water quality, the background concentrations can be compared to WHO MACs (Table 3). All background concentrations in the Great Zab, determined by the three methods used, are lower than WHO MACs. In some case, background concentrations are orders of magnitude lower, for example in the case of Cu and Zn (Table 3). The comparison to WHO MACs suggests that the background concentrations defined by this study can be viewed as very conservative if used as a guide for water quality based upon risks posed to human health. It is not suggested that the background concentrations derived here are adopted as a measure to protect human health; however, the concentrations quantified may act as valuable guide for identifying instances for anthropogenic pollution within the Great Zab catchment.
Variations in water chemistry within the catchment would mean that the instances of enrichment above background concentrations vary between waters from the Zagros, imbricated/high-fold and foothill zones. By way of example, comparison of Li, Ba, F− and NO3− concentrations, which have been shown to differ significantly between the three zones (Table 2), suggests that instances of elevation above background concentrations vary between zone and element (Fig. 9). Highest NO3− concentrations occur in the foothill zone, in waters from the Great Zab River itself, but also its tributaries, the Khazir River and the Gomal. NO3− concentrations in 7% of samples from foothill zone exceed the 95th percentile-defined background value, but samples from the Zagros and imbricated/high-fold zones do not. Conversely, Li and F− concentrations in the Zagros zone, in 9 and 7% of samples, respectively, exceed the 95th percentile-defined background value, with all samples in the two downstream zones falling below. These data further highlight that spatial trends in water chemistry, including the occurrence of relative elevation in concentrations, are likely to reflect both geogenic influences operating at larger spatial scales and site-specific conditions, particularly point sources associated with anthropogenic activity, that vary between different water quality parameters.
Conclusions
This study has conducted and presented data for the first regional survey of river water chemistry for the Great Zab River catchment, a major left-bank tributary of the River Tigris. Data analysis has shown that concentrations in river water of Ca, Li, Mo, Zn, F−, Cl−, SO42−and PO43− are significantly different during summer and winter sampling periods; however, there are variations in terms of when concentrations are highest for different elements. The Great Zab catchment can be divided into three major geological units, and the influence of bedrock geology upon surface water chemistry is evidenced by differences in elemental concentrations in waters sampled from the different units. In addition, whilst elemental concentrations in the Great Zab are below WHO MACs, a potential anthropogenic influence on water chemistry is identifiable through the location of highest elemental concentrations. The lower catchment, which is most highly populated, contains highest elemental concentrations and represents areas of concern for water resources management.
Background concentrations for the Great Zab catchment have been quantified using the 10th and 95th percentile concentrations and a regression analysis. These concentrations could be used as the basis for establishing water quality norms for the catchment and potentially the broader Kurdistan region. Comparison to WHO MACs, which provide human health-oriented guidelines for water quality, indicates that the use of a background concentration as a guide for water quality management would produce much more conservative and stricter quality limits. This suggests that there is a potentially significant difference between background concentrations, which may be used to identify instances of contamination within a catchment and guidelines that establish potential threats to human health. The use of a guideline such as a WHO MAC as a measure of water quality in water quality assessments may actually mask instances of poor water quality that are identified via the establishment of a catchment-wide background concentrations.
References
Abdulwahid S (2013) Water quality index of delizhiyan springs and Shawrawa River within Soran district, Erbil, Kurdistan Region of Iraq. J Appl Environ Biol Sci 3:40–48
Adams WJ, Chapman PM (2007) Assessing the hazards of metals and inorganic substances in aquatic and terrestrial systems. In: Proceeding from the workshop on hazard identification approach for metals and inorganic metal substances. Pensacola Beach, Florida. CRC Press, London
Alobaidy A, Abid H, Maulood B (2010) Application of water quality Index for assessment of Dokan lake ecosystem, Kurdistan region- Iraq. J Water Resour Prot 2:792–798
Alpers CN, Hunerlach MP, May JT, Hothem RL (2005) Mercury contamination from historical gold mining in California. United States Geological Survey, Sacremento, p 6
Berner EK, Berner RA (1996) Global environment: water, air and geochemical cycles. Prentice Hall, Upper Saddle River
Bird G, Brewer PA, Macklin MG, Nikolova M, Kotsev T, Mollov M, Swain C (2010) Contaminant-metal dispersal in mining-affected river catchments of the Danube and Maritsa drainage basins, Bulgaria. Water Air Soil Pollut 206:105–127
Chanpiwat P, Sthiannopkao S (2014) Status of metal levels and their potential sources of contamination in Southeast Asian rivers. Environ Sci Pollut Res 21:220–233
Davies BE (1983) A graphical estimation of the normal lead content of some British soils. Geoderma 29:67–75
Drever JI (1997) The geochemistry of natural waters, 3rd edn. Prentice Hall, Upper Saddle River
Fraters B, Bounmans LJM, Prins H (2001) Achtergrondconcentraites van 17 sporenmetalen in het groundwater van Nederland (in Dutch). RIVM, Bilthoven, The Netherlands. Report no. 711701017
Garbarino JR, Hayes HC, Roth DA, Antweiler RC, Brinton TI, Taylo HE (1995) Heavy metals in the Mississippi River. In: Meade RH (ed) Contaminants in the Mississippi River, 1987–1992. U.S. Geological Survey Circular 1133, Reston, Virgina
Gundersen P, Steinnes E (2001) Influence of temporal variations in river discharge, pH, alkalinity and Ca on the speciation and concentration of heavy metals in some mining polluted rivers. Aquat Geochem 7:173–193
Helsel DR, Hirsch RM (2002) Statistical methods in water resources techniques of water resources investigations. Book 4, chapter A3 U.S. Geological Survey
Ivanov VV (1996) Ekologicheskaya geokhimia elementov. Ekologia 1–6, Moscow, Russia (in Russian)
McDonald RI, Green P, Balk D, Fekete BM, Revenga C, Todd M, Montgomery M (2011) Urban growth, climate change, and freshwater availability. Proc Natl Acad Sci USA 108:6312–6317
McDonald RI, Weber K, Padowski J, Floerke M, Schneider C, Green PA, Gleeson T, Eckman S, Lehner B, Balk D, Boucher T, Grill G, Montgomery M (2014) Water on an urban planet: urbanization and the reach of urban water infrastructure. Glob Environ Change Hum Policy Dimens 27:96–105
Ministry of Planning (2011) Regional development strategy for Kurdistan region 2012–2016. Kurdistan Region Government (KRG), Erbil
Oste L, Zwolsman GJ, Klein J (2012) Methods to derive natural background concentrations of metals in surface water, and an application of two methods in a case study. Deltares report 1206111.005, Utrecht, p 104
Panno SV, Kelly WR, Martinsek AT, Hackley KC (2006) Estimating background and threshold nitrate concentrations using probability graphs. Groundwater 44:697–709
Peters A, Merrington G, Crane M (2012) Estimation of background reference concentrations for metals in UK freshwaters. Water Framework Directive - United Kingdom Technical Advisory Group, Edinburgh, p 49
Randall GW, Mulla DJ (2001) Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural processes. J Environ Qual 30:337–344
Reimann C, de Caritat P (1998) Chemical elements in the environment: factsheets for the geochemist and environmental scientist. Springer, Berlin
Reimann C, Garrett RG (2005) Geochemical background—concept and reality. Sci Total Environ 350:12–27
Saleh DK (2010) Stream gage descriptions and streamflow statistics for sites in the Tigris River and Euphrates River basins, Iraq. U.S. Geological Survey, Reston, p 147
Shareef KM, Muhamad SG (2008) Natural and drinking water quality in Erbil, Kurdistan. Curr World Environ 3:227–238
Shareef K, Muhamad S, Shekhani N (2009) Physical and chemical status of drinking water from water treatment plants on Great Zab River. J Appl Sci Environ Manag 13:89–92
Sissakian VK (2013) Geomorphology and morphometry of the Greater Zab River Basin, North of Iraq. Iraqi Bull Geol Min 9:21–49
Smith RA, Alexander RB, Schwarz GE (2003) Natural background concentrations of nutrients in streams and rivers of the conterminous United States. Environ Sci Technol 37:3039–3047
Stevanovic Z, Iurkiewicz A (2009) Groundwater management in northern Iraq. Hydrogeol J 17:367–378
Toma J (2011a) Limnological study in Dokan Lake, Kurdistan region of Iraq. J Environ Stud 6:1–12
Toma J (2011b) Physical and chemical properties and algal composition of Derbendikhan Lake, Sulaimania, Iraq. Curr World Environ 6:17–27
Vörösmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289:284–288
Acknowledgements
IAI acknowledges Ph.D. funding provided by the Kurdistan Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ismaiel, I.A., Bird, G., McDonald, M.A. et al. Establishment of background water quality conditions in the Great Zab River catchment: influence of geogenic and anthropogenic controls on developing a baseline for water quality assessment and resource management. Environ Earth Sci 77, 50 (2018). https://doi.org/10.1007/s12665-017-7190-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-7190-8