Abstract
The environment can play an important role in shaping how an animal behaves, and how well the animal performs in a particular environment can be influenced by early experiences. The tradition of releasing captive-reared juveniles into the wild in an effort to strengthen wild fish populations has often had little success owing to high post-release mortality. Fish reared under standard hatchery conditions are provided with fewer stimuli and they receive excess quantities of pellet food that are easy to handle and consume. Captive reared fish therefore appear to be under-stimulated and overfed. Several studies have demonstrated that simple structural enrichment in the rearing facilities promotes flexible behaviour compared to fish reared in plain, standard hatchery tanks. Less attention has been given to the effects of the diet. Here we use a cross-factored design to test the relative role of food ration and spatial enrichment on foraging behaviour. Our results show that fish from enriched environments, regardless of previous food-ration size, were more reluctant to start feeding on the first day in a novel arena. On day two and three, however, fish with prior experience of a low food ration showed greater foraging activity and efficiency than fish fed on full rations. On the second and third day, prior experience with enrichment was less important. We discuss how early feeding experience in combination with structural enrichment may contribute in producing fish that are better suited for release into the wild.
Similar content being viewed by others
Introduction
Following a decline in several important fish-stocks, particularly marine species, extensive restocking programmes have been undertaken, but often these have had limited success because of the high post-release mortality (Olla et al. 1994, 1998; Brown and Day 2002; Chan et al. 2003). For example, analysis of long-term data-sets from released larval cod along the Norwegian coast demonstrates little, or limited effect on populations, despite initial effects positively influencing the 0+ class (Salvanes et al. 1994; Svasand et al. 2000; Chan et al. 2003). There are, however, examples where larger juvenile cod have had higher post-release survival compared to smaller fish. This success may have been due to size-dependant predation or the fact the larger fish were reared in big, enclosed ponds which them gave a more natural rearing environment than conventional hatchery facilities (Kristiansen et al. 2000).
While in some cases failure of these programmes may be because the ecosystem is unable to support the large numbers of fish released, it is generally assumed that hatchery-reared fish are less fit for survival compared to fish that develop in the wild environment (Brown and Day 2002; Salvanes and Braithwaite 2006). As brood fish used for restocking purposes are commonly taken from local stock (so that local genetic adaptations are conserved), it is reasonable to assume that the major differences between wild and artificially reared fish are caused by the hatchery rearing environment. Fish reared for restocking are usually produced using commercial fish breeding techniques, where the focus is on rapid production of large numbers of individuals. The main difference between hatchery facilities and the wild is the homogenous nature of parameters that normally vary in a natural environment, such as temperature, salinity, light-conditions, fish density, feeding and the level of structural complexity.
It has been reported for many species, over several taxa, that early experience plays an important role in shaping adult behaviour (Sackett et al. 1999; Rosenzweig 2003; Knudsen 2004; Poirier et al. 2004; Braithwaite and Salvanes 2005). The kinds of early experience that can have an effect include social interactions, exposure to predators, experience of foraging on live prey and, more generally, interacting with structural complexity such as enrichment in the captive environment. A large body of literature has shown that introducing enrichment into the rearing environment induces behavioural changes. For example, in mammals, rats with structural enrichment display better problem solving capabilities (Rosenzweig and Bennett 1996), in birds the experience of a variety of airborne odours in early life predisposes pigeons to develop olfactory maps (Wiltschko et al. 1989). In fish shoaling responses are more varied in individuals reared with structural enrichment compared to fish reared in plain environments (Salvanes et al. 2007). These examples illustrate how the addition of enrichment can affect the development of the behavioural phenotype, and this may, in part, explain why hatchery fish behave differently to their wild counterparts.
To increase post-release survival one can try to mimic a natural environment in the hatchery. It is believed that a variable rearing environment may help with this by priming fish to behave in a more flexible and adaptive manner (Berejikian et al. 2001; Brown and Day 2002; Salvanes and Braithwaite 2006; Lee and Berejikian 2008). Most of the research investigating the effects of enrichment in fish rearing environments has focused on the role of structural enrichment. However, other stochastic environmental factors could be manipulated. For example, food availability in the wild is not evenly distributed, but rather it is patchy in time and space, unlike the hatchery where it is easy to catch pellet food that is continuously available.
In terms of post-release mortality, foraging and predation are considered to be major factors (Olla et al. 1994). Foraging is a risky activity, and animals need to be aware of the associated dangers—knowing when to be cautious is adaptive (Kelley and Magurran 2003). As such, the development of behaviour associated with the caution/boldness temperament axis is likely to have an impact on survival. A previous experiment in which cod were reared with variable enrichment produced fish that showed a greater propensity to explore a novel area, that were faster at recovering from stressor and that had a faster transition from pellets to live prey (Braithwaite and Salvanes 2005). In this study the timing of food availability was varied on a temporal and spatial basis, and this appeared to create fish that were bolder i.e. faster at emerging into a novel area (Braithwaite and Salvanes 2005). As yet, however, no studies have investigated the effect of how much food is available during rearing.
Here we use a cross factored design to determine the effects of a reduced food regime on hatchery fish reared with or without environmental enrichment. It is already known that the addition of physical enrichment into a hatchery environment generates fish with more adaptive behaviour. Our aim with this study was to determine whether the level of food availability interacts with physical enrichment to influence behaviour. For instance, fish on a lower ration may be forced to take more risks and develop bolder behaviour, but fish that experience variability in their physical environment as well as a lower food ration, may learn to be cautious under some circumstances and yet be bold under others.
Materials and methods
Fish
A total of 72 fish were tested with an additional 25 fish used for social stimuli. The social stimuli fish were used to encourage the test fish to feed while reducing the stress of social deprivation when single test fish were in observation tanks (Nordeide and Svasand 1990). As the fish were used for behavioural studies in aquaria, sub-samples were size-matched to standardize the fish-size relative to experimental aquaria and prey, but sampled randomly from all replicate rearing tanks. We used length as our size criteria, and the size of the fish selected were intermediate—i.e. not the longest or the shortest for any of the groups.
Fish were obtained as fertilized eggs from a commercial cod-farmer using local, wild-caught brood-stock (Bømlo, Western Norway). The eggs were spawned at the same day, and hatched on 4th of April 2006. At day one post hatching larvae were transported to the High Technology Centre in Bergen. Larvae were start-fed wild-caught, natural zooplankton and housed in plain, 1 × 1 × 0.6 m tanks from arrival until they were 90 days old. The cod were then introduced to commercially available dry food pellets (EWOS, MARIN) used throughout the rearing and during the experiment (see Table 1). The fish were kept on 12–12 h light regime, and flow-through seawater at 10 ± 0.5°C. The behavioural screening took place in November 2006.
All work was conducted with the approval of the departmental research ethics board at the Department of Biology, University of Bergen, under the Norwegian Veterinary Authorities site licence no 18.
Rearing environments
In July 2006, five enriched tanks (1 × 1 × 0.6 m) were set up as described in Braithwaite and Salvanes (2005), and a further five plain tanks were set up as controls. The introduction of pebbles, rocks and artificial kelp created the enrichment, while control environments consisted of standard, plain conventional rearing tanks. In the enriched tanks, rocks and pebbles covered approximately 65% of the bottom area. Light was provided by fluorescent tubes (40 W), placed 1.5 m above the water level. The tanks were flushed for debris every second day, and cleaned every week. Two of the enriched tanks and two of the plain were given reduced food-rations. This left three tanks with full rations in the enriched group and a further 3 in the plain group. The additional enriched and plain tanks in the full ration groups were reared for a different experiment, but some of those fish also took part in this experiment (i.e. fish in the full ration group were sampled from all three tanks for both plain and enriched treatments). Standard food rations were calculated from growth rates of wild cod (Hawkins et al. 1985), and adjusted for growth, using known food efficiency of the commercially available cod food (EWOS, MARIN) (See Table 1). Fish in the full ration food group (100%), were hand-fed with a full food-ration, delivered once a day, in the morning. The reduced-food groups (50%) were also fed a full ration, but every second day. To control for handling differences on the day without feeding, the tanks on reduced food-rations had a cup of sea-water added to their tank on days without feeding. These arrangements generated four treatment groups; (1) Enriched, 50% food ration, (2) Enriched, 100% food ration, (3) Plain, 50% food ration, (4) Plain, 100% food ration. The fish were reared under these conditions for 16 weeks before they were tested for behavioural differences.
Test-aquaria
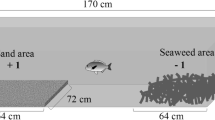
Screening took place in eight test-aquaria (95 l–70 × 38 × 36 cm); each sectioned into two equal partitions (Fig. 1), and separated by transparent glass. One side housed the test fish; the other side contained two social stimuli fish (Fig. 1). The stimuli fish were randomly sampled and distributed among the test-aquaria, and to avoid unnecessary stress from handling, they stayed in their designated experimental tanks during all replicate trials. The remaining nine stimuli fish were kept in a separate tank as replacements, to counter potential problems with mortality. All tanks were externally covered by black plastic on three sides, with the front covered by a removable, black plastic curtain that remained closed except for periods of filming or when we needed to observe the number of uneaten food-items. Each compartment had continuous flow-through of sea-water, from the same source, and with the same physical qualities as the water circulated in the rearing tanks.
Experimental protocol
A trial consisted of 3 days of observations, with eight parallel trials; two fish from each of the four treatment groups. For each trial, a single test fish were placed into one compartment, with a pair of social stimuli fish in the opposite compartment to reduce the effects of social deprivation when transferred to the experimental environment (Nordeide and Svasand 1990). Position of test fish in the left or right compartment of the test-aquaria was counterbalanced across trials. Thus, through the experiment, all groups were evenly distributed between left or right test compartments. The next morning (day 1) observations began. To stimulate the test fish into feeding on the novel prey, the stimuli fish were fed five live mysids (Praunus flexuosus) twice and at 180 and 90 min before the start of an observation. Prey were delivered through a hole in the tank lid, centrally positioned over each compartment. Test observations began by introducing five mysids into both the social stimuli fish and the test fish. For the first 15 min, the front curtain was raised and the tank was filmed (Canon MV700i VCR) to allow us to record foraging behaviour, movement and social preferences. After 15 min had elapsed, the front of the test-aquaria was covered with the black curtain, and the fish were left undisturbed, except when we counted the number of uneaten mysids at 45, 75 and 135 min after the beginning of each observation. All observations and counts were done by carefully lifting a bottom corner of the plastic cover in the front of the test-compartment corner. After the last count at 135 min, any uneaten mysids were removed from the test-aquaria.
On days 2 and 3, we followed the same procedure as described for day 1, but on these days we did not pre-feed the social stimuli fish, but fed them five mysids at the same time as the test fish. Thus, on days 2 and 3 the test fish and social stimuli fish were all fed five mysids at the start of the observations.
To quantify social interactions and activity, the compartment housing the test fish was divided with an imaginary line, creating a ‘near’ zone next to social stimuli fish (chosen as the section closest to the stimuli fish) when analysing the video of activity (Fig. 1). Activity and social preferences were scored manually from video recordings, using software from EthoLog (V 2.25) (Ottoni 2000). The activity was measured as the number of times a fish entered or left the ‘near’ zone (Fig. 1). The social preference was measured as time (s) spent in the near zone.
Statistical analysis
The probability of capturing a pursued prey was quantified from successful catches of individual prey at the set intervals. Social preferences were scored as time spent in the defined ‘near’ zone next to the social stimuli fish. Activity was scored as the number of times the fish moved in and out of the near zone to be close to the social stimuli fish.
All data were analysed using R 2.11.1 (Crawley 2007; R Development Core Team 2010). We used generalized linear mixed effects models (glmmPQL) (Zuur et al. 2009; Venables and Ripley 2002), with binomial error distribution to statistically assess the importance of rearing environment (enriched and plain), ration size (100% ration and 50% ration) and trial day (1, 2 and 3) on the probability of capturing prey. A glmmPQL with Poisson error distribution was used to test for the effects of rearing environment, ration size and day on the number of times individuals moved into or out of the ‘near’ zone, and on number of attacks against mysids (Zuur et al. 2009; Venables and Ripley 2002).
To test if time spent in the ‘near’ zone varied, we used a linear mixed effect model (lme), assuming a normal error distribution (Zuur et al. 2009). All of these analyses included rearing environment, ration size and day as main effects and individual ID as a random effect, and assuming a first-order autocorrelation structure in this repeated measures analysis. The variable day was treated as an ordered categorical variable. We included all main effects and interaction terms in the initial model, which was then simplified by sequentially removing all non-significant terms to achieve the minimal adequate model. The analysis of number of attacks against mysids the first 15 min of each day showed only an effect of day and food level and the model had no significant interactions.
To test for length, weight and condition factor depending on food level and rearing background (enriched vs. plain), we used a two-way ANOVA.
Results
Fish that experienced a 50% food ration increased their probability of capturing prey more rapidly over the 3 experimental days than fish that experienced a 100% food ration (glmmPQL; t = 2.445, p = 0.015, Fig. 2). On day 1, there was no difference in prey capture success between 50% and 100% food ration fish (glmmPQL; t = 0.269, p = 0.789), but plain-reared fish had a higher probability of capturing pursued prey than enriched fish (glmmPQL; t = 2.796, p = 0.007, Fig. 2).
The number of attacks increased over days (glmmPQL; t = 3.924, p < 0.001), and 50% food ration fish were more active in pursuing prey than 100% food ration fish (glmmPQL; t = 2.974, p = 0.004).
All treatment groups spent a similar amount of time in the ‘near’ zone next to the social stimuli fish (lme: rearing environment F 1,68 = 0.006, p = 0.941; food ration, F 1,68 = 0.017, p = 0.896; day, F 2,134 = 1.032, p = 0.359). Fish activity (the number of times a fish entered or left the ‘near’ zone next to the social stimuli fish) increased over days (glmmPQL; t = 3.684, p < 0.001; Fig. 3). Plain fish were consistently more active than enriched fish (glmmPQL; t = 2.291, p = 0.025; Fig. 3).
Predicted activity level estimated as the number of times the fish crossed the border between the far and the near zone (next to the social stimuli fish) as a function of experimental day and rearing environment (enriched and plain) the first 15 min within each day. The lines represent predicted values from the glmmPQL
There were no significant differences in weight, length and C-factor between the plain and enriched groups. Fish from 100% food groups tended to be longer (ANOVA; F = 2.94; p = 0.091, Table 2a), and they weighed significantly more (ANOVA: F = 4.58; p = 0.036 Table 2b) and had a higher C-factor (ANOVA; F = 8.23; p = 0.005, Table 2c).
Discussion
When cod were introduced to novel, live prey in the test environment, fish with experience of enrichment were more reluctant to start foraging compared to fish from plain tanks. This was the case regardless of the feeding ration they had experienced previously. On days 2 and 3 of the experiment, however, feeding ration had a bigger effect with the 50% fed fish, showing higher clearance rates of live mysids than 100% ration fed fish, regardless of habitat background. Together these results suggest that in a novel situation, the experience with enrichment has an immediate effect on foraging behaviour, seen here as an initial reluctance to forage on a novel type of prey. However, having been fed on a 50% limited ration also influences foraging motivation and how efficiently prey is consumed. We observed that fish reared in plain tanks generally had higher levels of activity, moving more frequently in and out of the ‘near’ zone, close to the clear partition separating the test fish from the visual stimuli fish. Previous research has found that structural enrichment promotes flexible behaviour in hatchery-reared fish in terms of foraging, aggression, shoaling, stress-recovery and social learning (Salvanes and Braithwaite 2005; Salvanes et al. 2007; Strand et al. 2010); all of these factors that seem relevant to the boldness/caution complex.
In the present study, experience with structural enrichment in early life appears to influence the development of cautious behaviour by creating less bold, more cautious individuals as seen on day one. In a previous study we found enrichment to affect shoaling responses relative to the test-environment (Salvanes et al. 2007). Fish from enriched rearing environments were more flexible and switched between shoaling in the simple plain test environment to a more individual behaviour in a structurally complex environment, while fish reared in plain tanks shoaled regardless of the environment. This can be explained as a shift towards territorial behaviour in the fish from the enriched environment, while fish reared in plain, open-water environment show a more fixed shoaling mode irrespective of test environment. Salvanes and Braithwaite (2005) found enrichment to produce cod with asymmetries in aggressive behaviour, and also to have a higher use of shelter after attacks. Enriched fish were less likely to flee from attacks, and also directed their attacks more towards fish from the plain environment, despite the plain fish being significantly larger, suggesting a territorial behaviour as well as a capacity to assess the other fish in a conflict.
Shoaling is a group behaviour related to foraging as well as anti-predator defence and there is a certain trade-off between the relative safety in the group, and the increased foraging competition (Hoare et al. 2000).
Differences in shoaling behaviour may affect the foraging on several levels. For example, fish from the plain environment may have adapted to more direct competition with shoaling conspecifics in the open water environment. Fish from enriched environments, however, are more likely to enter a territorial mode with a more individual behaviour than the plain controls with a more fixed shoaling mode, and this may affect activity levels and how it searches for, and approaches prey. Non-territorial individuals from plain environments may therefore, through both higher activity and different search-modes, have higher encounter-rates of prey in the experimental area, while the fish with territorial experience to larger degree may act as a “sit and wait” predator, displaying less activity and appearing to be more cautious. Another factor in an enriched environment is that left-over food on the bottom opens up the possibility to forage among the enrichment rocks and pebbles, thereby creating an alternative strategy to surface feeding. The continuous competition with conspecifics in surface-feeding may favour aggressive, competitive feeding behaviour in the plain tanks, as opposed to fish from the enriched rearing environments that may avoid conflicts by adjusting to a bottom-feeding strategy. Thus, both the social differences and the structural environment must be expected to affect the way the fish search for, and approach their prey.
Consequently, we suggest that the observed differences in activity, boldness and foraging behaviour seen between fish with and without enrichment result from fish having to adapt to different levels of competition and foraging responses appropriate to their respective rearing environments.
The level of food ration also played a role, and appeared to interact with boldness, as the fish from the 50% food ration groups displayed more active and efficient foraging behaviour from day two. While earlier work indicates a role for structural enrichment, little is known about how food availability affects the development of foraging behaviour in hatchery fish. One study that partially addressed this, showed that juvenile cod exposed to a variable feeding regime (full ration), were quicker to transfer to a live prey diet (Braithwaite and Salvanes 2005). Our present study demonstrates a novel result; that cod reared with a low ration diet were also better at consuming novel live prey.
Several factors may explain the observed differences in foraging efficiency, and factors like feeding motivation, hunger state and the size-relationship between predator and prey should be considered. Size-difference between experimental fish and the prey and between test groups could not explain our results. We selected fish such that they were within an approximate length range that was appropriate for both the prey and aquarium size. As such, motivation related to the profitability of prey (based on prey-size), or differences in the relative size of the search area were minimized. Consequently, differences in foraging motivation may be affected by different food-administrations, i. e differences in time between feeding, rather than amount of food. We could not ignore, however, potential differences in metabolic rates or hunger-state that could be owing to reduced prior food-regimes. This could have contributed to a difference in motivation and foraging efficiency.
Motivation and activity are essential parts in learning processes, and increasing success-rates must be expected to correlate to number of foraging attempts. Thus, the increased foraging efficiency suggests that the 50% food ration group learned to catch and handle the novel prey at a faster rate than the 100% food ration group, but that this increase in learning rate may arise because of motivation and experience rather than better cognitive abilities.
Both structural enrichment and pre-training fish with live prey has been suggested to increase post-release survival in fish produced for release. Some studies have investigated feeding live-prey to hatchery-reared fish just prior to their release. Such research has proved inconclusive as fish with prior experience of live prey may feed well on the live prey they were trained with (Ellis et al. 2002), but they may not necessarily forage so well when introduced to other novel prey species (Massee et al. 2007). One experiment which investigated the effect of prior exposure to live prey in combination with enriched environments in juvenile Atlantic salmon (Salmo salar L.), did report that fish can benefit from such a combination (Brown et al. 2003), thus presenting the rearing enrichment itself as a factor in the development of foraging behaviour. Thus, we suggest that if fish are reared for release, a low food ration combined with an enriched rearing environment may provide the fish with more relevant experience to prepare the fish for the wild.
While reduced food-rations appear to have a positive effect on foraging behaviour, they should not be reduced to a level that affects growth to a degree that may induce compensatory growth responses. Fish, in general have a strong ability to compensate for slow growth by bursts of rapid growth (Ali et al. 2003), often linked with negative long term effects on fitness (Metcalfe and Monaghan 2001). Consequently, releasing fish during a period of compensatory growth may counter the otherwise beneficial effects that variable food regime and environmental enrichment may provide.
Previously it has been suggested that providing a diet that reflects naturally occurring food would be one way to improve survival in hatchery-reared fish (Maynard et al. 1996). But the reality is that such an approach will be impossible for large-scale production of fish, because rearing sufficient quantities of live invertebrate prey is too costly and too labour intensive. The results we report here, however, suggest that an alternative approach may be to vary food rations in combination with structural enrichment in otherwise standard rearing facilities. Together, these two methods may provide a feasible way of rearing hatchery fish with increased post-release survival.
References
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190
Berejikian BA, Tezak EP, Riley SC, LaRae AL (2001) Competitive ability and social behaviour of juvenile steelhead reared in enriched and conventional hatchery tanks and a stream environment. J Fish Biol 59:1600–1613
Braithwaite VA, Salvanes AGV (2005) Environmental variability in the early rearing environment generates behaviourally flexible cod: implications for rehabilitating wild populations. P Roy Soc B-Biol Sci 272: 1107–1113.
Brown C, Day RL (2002) The future of stock enhancements: Lessons for hatchery practice from conservation biology. Fish Fish 3:79–94
Brown C, Davidson T, Laland K (2003) Environmental enrichment and prior experience of live prey improve foraging behaviour in hatchery-reared Atlantic salmon. J Fish Biol 63:187–196
Chan KS, Stenseth NC, Kittilsen MO, Gjosaeter J, Lekve K, Smith T, Tveite S, Danielssen D (2003) Assessing the effectiveness of releasing cod larvae for stock improvement with monitoring data. Ecol Appl 13:3–22
Crawley MJ (2007) The R Book. R: a language and environment for statistical computing. Wiley, Vienna
Ellis T, Hughes RN, Howell BR (2002) Artificial dietary regime may impair subsequent foraging behaviour of hatchery-reared turbot released into the natural environment. J Fish Biol 61:252–264
Hawkins AD, Soofiani NM, Smith GW (1985) Growth and feeding of juvenile cod (Gadus morhua L). J Conseil 42: 11–32
Hoare DJ, Krause J, Peuhkuri N, Godin JGJ (2000) Body size and shoaling in fish. J Fish Biol 57:1351–1366
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fish 4:216–226
Knudsen EI (2004) Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16:1412–1425
Kristiansen TS, Ottera H, Svasand T (2000) Size-dependent mortality of juvenile reared Atlantic cod released in a small fjord. J Fish Biol 56:792–801
Lee JSF, Berejikian BA (2008) Effects of the rearing environment on average behaviour and behavioural variation in steelhead. J Fish Biol 72:1736–1749
Massee KC, Kim J, Berejikian BA, Hardy RW (2007) Prey selection and efficiency of naive and experienced juvenile sockeye salmon. J Fish Biol 70:1213–1223
Maynard DJ, McDowell GC, Tezak EP, Flagg TA (1996) Effect of diets supplemented with live food on the foraging behavior of cultured fall chinook salmon. Prog Fish Cult 58: 187–191
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Nordeide JT, Svasand T (1990) The behavior of wild and reared juvenile cod, (Gadus-morhua L). Towards a potential predator. Aquaculture and Fisheries Management 21: 317–326
Olla BL, Davis MW, Ryer CH (1994) Behavioural deficits in hatchery-reared fished: potential effects on survival following release. Aquacult Fish Manage 25:19–34
Olla BL, Davis MW, Ryer CH (1998) Understanding how the hatchery environment represses or promotes the development of behavioral survival skills. B Mar Sci 62: 531–550
Ottoni EB (2000) EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Meth Instrum Comput 32:446–449
Poirier R, Chichery R, Dickel L (2004) Effects of rearing conditions on sand digging efficiency in juvenile cuttlefish. Behav Process 67: 273–279
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rosenzweig MR (2003) Effects of differential experience on the brain and behavior. Dev Neuropsychol 24:523–540
Rosenzweig MR, Bennett EL (1996) Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 78:57–65
Sackett GP, Novak B, Kroeker R (1999) Early experience effects on adaptive behavior: theory revisited. Ment Retard Dev Disabil Res Rev 5:30–40
Salvanes AGV, Braithwaite VA (2005) Exposure to variable spatial information in the early rearing environment generates asymmetries in social interactions in cod (Gadus morhua). Behav Ecol Sociobiol 59:250–257
Salvanes AGV, Braithwaite VA (2006) The need to understand the behaviour of fish reared for mariculture or restocking. ICES J Mar Sci 63:346–354
Salvanes AGV, Giske J, Nordeide JT (1994) Life-history approach to habitat shifts for coastal cod, Gadus morhua L. Aquac Fish Manage 25:215–228
Salvanes AGV, Moberg O, Braithwaite VA (2007) Effects of early experience on group behaviour in fish. Anim Behav 74:805–811
Strand DA, Utne-Palm AC, Jakobsen PJ, Braithwaite VA, Jensen KH, Salvanes AGV (2010) Enrichment promotes learning in fish. Mar Ecol Prog Ser 412:273–282. doi:10.3354/meps08682
Svasand T, Kristiansen TS, Pedersen T, Salvanes AGV, Engelsen R, Naevdal R, Nodtvedt M (2000) The enhancement of cod stocks. Fish Fish 1:173–205
Venables W, Ripley B (2002) Modern Applied Statistics with S. Springer, New York. ISBN: 0-387-95457-0
Wiltschko R, Schops M, Kowalski U (1989) Pigeon homing-wind exposistions determines the importance of olfactory input. Naturwissenschaften 76:229–231
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank Frank Midtøy and David Strand for their help in setting up and running the experiment, and members of the Aquatic Behavioural Group for valuable comments and discussions. This work was supported by the Department of Biology, University of Bergen and The Norwegian Research Council (Grant: 177888/v40)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 29.0 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Moberg, O., Braithwaite, V.A., Jensen, K.H. et al. Effects of habitat enrichment and food availability on the foraging behaviour of juvenile Atlantic Cod (Gadus morhua L). Environ Biol Fish 91, 449–457 (2011). https://doi.org/10.1007/s10641-011-9803-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-011-9803-5