Abstract
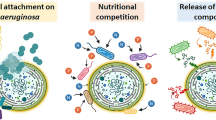
The ecological influence of cyanotoxins on aquatic biota remains unclear despite the numerous published references on toxicological and sanitary problems related with cyanophyte proliferation. The effects of microcystins and cyanophyte extracts on the photosynthesis of the algae that belong to two taxonomic groups, Rhodophyta and Bacillariophyta, were studied in an attempt to elucidate their role in the intraspecific competence and physiognomy of fluvial communities. The data showed that both cyanobacteria extracts and pure microcystin-LR affected the photosynthetic activity of all the tested organisms, diatoms (Fistulifera pelliculosa, Gomphonema parvulum, Nitzschia frustulum and Stephanodiscus minutulus) and red algae (Chroothece richteriana) at environmentally relevant concentrations. Effects varied with strains and time, and promoted or inhibited photosynthesis. The microcystins and the other compounds present in cyanobacteria extracts may explain the competence effects observed in nature, especially in calcareous environments where they predominate, and after disturbing events like heavy rains or floods, which may destroy cyanophyte mats and release toxic or inhibitory compounds in a seasonal scale pattern.


Similar content being viewed by others
References
Aboal M, García-Fernández ME, Roldán M, Whitton BA (2014) Ecology, morphology and physiology of Chroothece richteriana (Rhodophyta, Stylonematophyceae) in the highly calcareous Río Chícamo, south-east Spain. Eur J Phycol 49(1):83–96
Aboal M, Puig MA (2005) Intracellular and dissolved microcystin in reservoirs of the river Segura basin, Murcia, SE Spain. Toxicon 45(4):509–518
Aboal M, Puig MA, Asencio AD (2005) Production of microcystins in calcareous Mediterranean streams: the Alhárabe, Segura river Basin in S.E. Spain. J Appl Phycol 17(3):231–243
Aboal M, Puig MA, Mateo P, Perona E (2002) Implications of cyanophyte toxicity on biological monitoring of calcareous streams in north-east Spain. J Appl Phycol 14(1):49–56
Aboal M, Puig MA, Ríos H, López-Jiménez E (2000) Relationship between macroinvertebrate diversity and toxicity of Cyanophyceae (Cyanobacteria) in some streams from eastern Spain. J Appl Phycol 14(1):49–56
Asencio AD (2013) Determination of microcystins in reservoirs of different basins in a semiarid area. J Appl Phycol 25:1753–1762. doi:10.1007/s10811-013-0025-4
Azevedo SM, Carmichael W, Jochimsen E, Rinehart K, Lau S, Shaw G, Eaglesham G (2002) Human intoxication by microcystins during renal dialysis treatment in Caruaru/Brazil. Toxicology 181/182:441–446
Backer LC, Landsberg JH, Miller M, Keel K, Taylor TK (2013) Canine cyanotoxins posionings in the United States (1920s-2012): review of suspected and confirmed cases from three data sources. Toxins 5(9):1597–1628
Bengston-Nash SM, Schreiber U, Ralph PJ, Müller JF (2005) The combined SPE: ToxY-PAM phytotoxicity assay; application and appraisal of a novel biomonitoring tool for the aquatic environment. Biosens Bioelectro 20:1443–1451
Campos A, Araújo P, Pinheiro C, Azevedo J, Osório H, Vasconcelos V (2013) Effects on growth, antioxidant enzyme activity and level sof extracelular proteins in the Green alga Chlorella vulgaris exposed to crude cyanobacterial extracts and pure microcystin and cylindrospermopsin. Ecotoxicol Environ Saf 94:45–53
Carvalho LR, Costa-Neves A, Conserva GAA, Brunetti RL, Hentschke GS, Malone CFS, Torres LMB, Sant’Anna CL, Rangel M (2013) Biologically active compounds from cyanobacteria extracts: in vivo and in vitro aspects. Revista Brasileira de. Farmacognosia 23(3):471–480
Chauhan VS, Marwah JB, Bagchi SN (1992) Effect of anantibiotic from Oscillatoria sp. on phytoplankters, higher plantsand mice. New Phytol 120:251–257
Chorus I (Ed) (2002) Cyanotoxins. Occurrence, causes and consequences. Springer, Berlin-Heidelberg
Codd GA (1996) Awareness of Cyanobacterial or Algal Blooms at the Premonstratensian Monastery of the Green Loch, Soulseat Scotland, from the Twelfth Century, and Cattle Poisonings Attributed to Cyanobacterial Hepatotoxins at this location Eight Hundred Years Later. In: The Intergovernmental Oceanographic Commission of UNESCO, (1996) Harmful algae news. An IOC Newsletter on toxic algae and algal blooms No. 15
Codd GA, Azevedo SMFO, Bagchi SN, Burch MD, Carmichael WW, Harding WR, Kaya K, Utkilen HC (2005) A Global Network for Cyanobacterial Bloom and Toxin Risk Management. Initial situation Assessment and Recommendations. IHP-VI. Technical Documenrs in Hydrology, n° 76, UNESCO, Paris
Codd GA, Bell S, Kaya K, Ward C, Beattie K, Melcalf J (1999) Cyanobacterial toxins, exposure routes and human health. Eur J Phycol 34(4):405–415
Cosgrove J, Borowitzka M (2006) Applying pulse amplitude modulation (PAM) fluorometry to microalgae suspensions: stirring potentially impacts fluorescence. Photosynth Res 88:343–350
Edwards C, Beattie KA, Scrimgeour CM, Codd GA (1992) Identification of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Inshen, Scotland. Toxicon 30:1165–1175
Eigemann F, Vanormelingen P, Hilt S (2013) Sensitivity of the green alga Pediastrum duplex Meyen to allelochemicals is strain-specific and not related to co-occurrence with allelopathic macrophytes. PLoS ONE 8(10):e78463. doi:10.1371/journal.pone.0078463
Faassen EJ, Lürling M (2013) Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar Drugs 11:2643–2654
Fukami K, Nishijima T, Ishida Y (1997) Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia 358:185–191
Fukami K, Yuzawa A, Nishijima T, Hata Y (1992) Isolation and properties of a bacterium inhibiting the growth of Gymnodinium nagasakiense. Nippon Suisan Gakkaishi 58:1073–1077
Gallacher S, Flynn KJ, Franco JM, Brueggemann EE, Hines HB (1997) Evidence for production of paralytic shellfish toxins bybacteria associated with Alexandrium spp. (Dinophyta) in culture. Appl Environ Microbiol 63:239–245
Gantar M, Berry JP, Thomas S, Wang M, Perez R, Rein KS, King G (2008) Allelopathy activity among Cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol Ecol 64(1):55–64. doi:10.1111/j.1574-6962.2008.00439.x
Genty B, Brintais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990(1):87–92
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
Gutiérrez-Praena D, Jos A, Pichardo S, Moreno IM, Cameán AM (2013) Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: a review. Food Chem Toxicol 53:139–152
Hagmann L, Jüttner F (1999) Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett 36:6539–6542
Indejir K, Dakshini MM (1994) Algal allelopathy. Bot Rev 60(2):26–60
Jones GJ, Bourne DG, Blakeley RL, Doelle H (1994) Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat Toxins 2:228–235
Jos A, Pichardo S, Prieto AI, Repetto G, Vázquez CM, Moreno I, Cameán AM (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat Toxicol 72(3):261–271
Kang YH, Kim JD, Kim BH, Kong DS, Han MS (2005) Isolation and characterization of a bio-agent antagonistic to diatom, Stephanodiscus hantzschii. J Appl Microbiol 98:1030–1038
Kaya K, Mahakhant L, Keovara L (2002) Spirodesin, a novel lipopeptide from the cyanobacterium Anabaena spiroides that inhibits cell growth of the cyanobacterium Microcystis aeruginosa. J Nat Prod 65:920–921
Kearns KD, Hunter MD (2001) Toxin-producing Anabaena flos-aquae induces settling of Chlamydomonas reinhardtii, a competing motile alga. Microbiol Ecol 42:80–86
Keating KI (1978) Blue-green algal inhibition of diatom growth: transition from mesotrophic to eutrophic community structure. Science 199:971–973
Krammer K, Lange-Bertalot H (1986) 2/1. Bacillariophyceae. 1. Teil: naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart, p 876. 206 Tafeln mit 2976 Figuren
Krammer K, Lange-Bertalot H (1988) 2/2. Bacillariophyceae. 2. Teil: Bacillariaceae. Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart, p 596. 184 Tafeln mit 1914 Figuren
Krammer K, Lange-Bertalot H (1991a) 2/3. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart, p 576. 166 Tafeln mit 2180 Figuren
Krammer K, Lange-Bertalot H (1991b) 2/4. Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds.) Süβwasserflora von Mitteleuropa. G. Fischer Verlag, Stuttgart, p 437. 88 Tafeln mit 2048 Figuren
Leão PN, Pereira AR, Liu WT, Ng J, Pevzner PA, Dorrestein PC, König M, Vasconcelos VM, Gerwick WH (2010) Synergistic allelochemicals from a freshwater cyanobacteria. PNAS 107(25):11183–11188
Leão PN, Vasconcelos MTSD, Vasconcelos VM (2009) Alleopathic activity of cyanobacteria on green microalgae at low cell densities. Eur J Phycol 44(3):347–355
Leflaive J, Ten-Hage L (2007) Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshwater Biol 52:199–214
Legrand C, Rengefors K, Fistarol GO, Grabeli E (2003) Allelopathy in phytoplankton – biochemical, ecological and evolutionary aspects. Phycologia 42(4):406–419
Máthé C, M-Hamvas M, Vasas G (2013) Microcystin-LR and Cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar Drugs 11(10):3689–3717
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420
Mogelhoj MK, Hansen PJ, Henriksen P, Lundholm N (2006) High pH and not allelopathy may be responsible for negative effects of Nodularia spumigena on other algae. Aquat Microb Ecol 43:43–54
Molish H (1979) Der Einfluss einer Pflanze auf die andere –Allelopathie. Fischer, Jena
Moreno IM, Pichardo S, Moyano R, Gustavo González AG, Cameán AM (2010) Determination of microcystins in biological samples from freshwater fish. Inter J Environ Anal Chem 90(13):1000–1013
Moreno I, Repetto G, Cameán A (2003) Interés toxicológico de las microcistinas. Rev Toxicol 20:59–165
Peleato ML (2011) Las cianobacterias: cooperación versus competencia. Real Academia de Ciencias Exactas, Físicas, Químicas y Naturales de Zaragoza. Zaragoza
Perron MC, Qiu B, Boucher N, Bellemare F, Juneau P (2012) Use of chlorophyll a fluorescence to detect the effect of microcystins on photosynthesis and photosystem II energy fluxes of green algae. Toxicon 59:567–577
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins with reference to Microcystin-LR, in aquatic ecosystems. Environ Toxicol 17:407–413
Pinheiro C, Azevedo J, Campos A, Loureiro S, Vasconcelos V (2013) Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 705:27–42
Ray S, Bagghi SN (2001) Nutrients and pH regulate algicide accumulation in cultures of the cyanobacterium Oscillatoria laetevirens. New Phytol 149:455–460
Rice EL (1979) Allelopathy –an update. Bot Rev 45:15–109
Ríos V, Moreno I, Prieto AI, Puerto M, Gutiérrez-Praena D, Cameán AM (2013) Analysis of MC-LR and MC-RR in tissue from freshwater fish (Tinca tinca) and crayfish (Procambarus clarkii) in tench ponds (Cáceres, Spain) by liquid chromatography–mass spectrometry (LC–MS). Food Chem Toxicol 57:170–178
Sabater S, Guasch H, Muñoz I (2006) Hydrology, light and the use of organic and inorganic materials as structuring factors of biological communities in Mediterranean streams. Limnetica 25(1–2):335–348
Schlegel I, Doan NT, de Chazal N, Smith GD (1999) Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and Cyanobacteria. J Appl Phycol 10:471–479
Schreiber U (2001) Dual-channel phtosynthesis yield analyzer ToxY-PAM: handbook of operation. Heinz Walz GmbH, Effeltrich, Germany
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995) Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust J Plant Physiol 22:209–220
Schreiber U, Mueller JF, Haugg A, Gademann R (2002) New type of dual-channel PAM chlorophyll fluorometer for highly sensitive water toxicity biotests. Photosynth Res 74(3):317–330
Sedmak B, Elersek T (2006) Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb Ecol 51:508–515
Sivonen K, Carmichael WW, Namikoshi M, Rinehart KL, Dahlem AM, Niemela SI (1990) Isolation and Characterization of Hepatotoxic Microcystin Homologs from the Filamentous Freshwater Cyanobacterium Nostoc sp. Strain 152. Appl Env Microbiol 56(9):2650–2657
Sliwinska S, Latala A (2012) Allelopathy effects of cyanobacterial filtrates on Baltic diatom. Contemporary Trends in Geoscience 1:103–107
Smayda TJ (1997) Harmful algal blooms: their ecophysiology and genera relevance to phytoplankton blooms in the sea. Limnol Oceanogr 42(5):1137–1153
Starodub NF, Katzev AM, Starodub VM, Levkovetz IA, Goncharuk VV, Klimenko NA, Shmir’ova AN, Piven NV, Dzantijev BB (2005) Biosensors for water quality monitoring. modern tools and methods of water treatment for improving living standards. NATO Science Series 48:51–70
Stewart I, Seawright AA, Shaw GR (2008) Cyanobacterial poisoning in livestock, wild mammals and birds-an overview. Adv Exp Med Biol 619:613–637
Suikkanen S, Fistarol GO, Granéli E (2004) Allelopathic effects of the Baltic cyanobacteria Nodularia spumigena, Aphanizomenon flos-aquae and Anabaena lemmermannii on algal monocultures. J Exp Mar Biol Ecol 308:85–10
Suikkanen S, Fistarol GO, Granéli E (2005) Effects of cyanobacterial allelochemicals on a natural plankton community. Mar Ecol Prog Ser 287:1–9
Svircev Z, Krstic S, Miladinov-Mikov M, Baltic V, Vidovic M (2009) Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J Environ Sci Health C 27:36–55
Thomas AD, Saker HL, Norton HJ, Olsen RD (1998) Cyanobacterium Cylindrospermopsis raciborskii as a probable cause of death in cattle in northern Queensland. AusVetJ 76:592–594
Tillett D, Dittmann E, Erhard M, von Dohren H, Borner T, Neilan BA (2000) Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptidepolyketide synthetase system. Chem Biol 7:753–764
Valdor R, Aboal M (2007) Effects of living cyanobacteria, cyanobacterial extracts and pure microcystins on growth and ultrastructure of microalgae and bacteria. Toxicon 49:769–779
Von Elert E, Jüttner F (1997) Phosphorus limitation and not light controls the extracellular release of allelopathic compoundsby Trichormus doliolum (Cyanobacteria). Limnol Oceanogr 42:1796–1802
Weissbach A, Tillmann U, Legrand C (2010) Allelopathic potential of the dinoflagellate Alexandrium tamarense on marine microbial communities. Harmful Algae 10:9–18. doi:10.1016/j.hal.2010.05.007
Whitton BA, Potts M (2000) The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, p 669
Acknowledgements
We thank the Seneca Foundation of the Murcia Region (05762/PI/07), the Consejería de Universidades, Empresa e Investigación of the Murcia Region (PEPLAN-S5), the Science and Innovation Spanish Ministry (CGL2009-09563) for their support, and the PASPA-DGAPA-UNAM programme for support to the second author for a sabbatical stay. We also wish to thank V Osorio and MÀ Puig for their help with the statistical analysis, MD Pardo for the HPLC analysis, and H Warburton for her assistance with the English version of the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human and animal rights
This article does not contain any studies by any of the authors performed with human participants or animals.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Rights and permissions
About this article
Cite this article
García-Espín, L., Cantoral, E.A., Asencio, A.D. et al. Microcystins and cyanophyte extracts inhibit or promote the photosynthesis of fluvial algae. Ecological and management implications. Ecotoxicology 26, 658–666 (2017). https://doi.org/10.1007/s10646-017-1798-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1798-z