Abstract
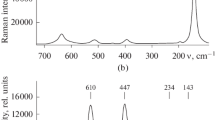
Biocompatibility of the surfaces of titanium dental implants can be improved by covering them with calcium phosphate crystals, which makes the surface bioreactive. Possibly the most effective bioreactive foreign material that improves osteointegration and adsorption/binding of extracellular proteins and structural proteins is crystalline octacalcium phosphate {2×[Ca4H(PO4)3·2.5H2O] or Ca8(HPO4)2(PO4)4·5H2O, OCP}. In this work the building up of OCP crystals on the surface of TiO2 anatase is examined in the process of heterogeneous nucleation from constant-composition solutions of CaCl2 and KH2PO4 at constant pH (pH 6.8) and ionic strength (I=0.05 M), in dense titania suspensions. Constant relative supersaturation with regard to the calcium phosphate formation was maintained by the controlled addition of the reagent solutions, according to the desired speed of crystallization. The surface saturation value of calcium ion adsorption was measured by detecting the pH decrease during CaCl2 addition in a separate experiment. The OCP crystallization was also conducted on the surface of an evaporated titanium layer, and on titanium metal disks. The surface of the disks was modified by the laser ablation method in order to increase the oxide layer thickness. Calcium phosphate crystals formed on the surface of the modified titanium disks, but not in an appreciable amount on the surface of the evaporated titanium layer.









Similar content being viewed by others
References
Lacefield WR (1999) Adv Dent Res 13:21
Wang RR, Fenton A (1996) Prosthodontics 27:401
Kasuga T, Mizuno T, Watanabe M, Nogami M, Miinomi M (2001) Biomaterials 22:577
Wang CX, Chen ZO, Wang M, Lin ZY, Wang PL (2001) J Biomed Mater Res 55:587
de Groot K, Geesink R, Klein C (1987) J Biomed Mater Res 21:1375
Ban S, Maruno S, Arimoto N, Harada A, Hasegawa J (1997) J Biomed Mater Res 36:9
Wu W, Nancollas GH (1997) Langmuir 13:861
Combes C, Rey C, Fresche M (1998) Colloids Surf B 11:15
Li P, Kangasniemi I, de Groot K (1994) J Am Ceram Soc 77:1307
Wu W, Nancollas GH (1998) J Colloid Interface Sci 199:206
Areva S, Linden M (2003) Bioceram 15 Key Engi Mater 240:465
Shibata Y, Miyazaki T (2002) J Dent Res 81:841
Pach L, Komarneni S (1999) Mater Res Bull 34:1859
Feng QL, Cui FZ, Wang H, Kim TN, Kim JO (2000) J Cryst Growth 210:735
Es-Soumi M, Zimehl R, Fisher-Brandies H (1999) Colloid Polym Sci 277:382
Heughebaert JC, Zawacki SJ, Nancollas GH (1983) J Cryst Growth: 63:83
Liu XY (2000) Langmuir 16:7337
(a) Thomson MB, Nancollas GH (1978) Science 200:1059; (b) Koutsoukos PG, Amjad Z, Tomson MB, Nancollas GH (1980) J Am Chem Soc 102:1553; (c) Amjad Z, Koutsouskos PG, Nancollas GH (1984) J Colloid Interface Sci 101:250
James RO, Parks GA (1982) Surf Colloid Sci 12:119
Huang C-P, Stumm W, (1973) J Colloid Interface Sci 43:409
Brown WE, Smith JP, Lehr JR, Fraizer AW (1962) Nature 196:1048
Gallardo-Amores JM, Armaroli T, Ramis G, Finocchio E, Busca G (1999) Appl Catal B 22:249
Brown G (1980) In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification, Mineralogical Society, London, pp 361–411
Bereznai M, Pelsőczi I, Tóth Z, Turzó K, Radnai M, Bor Z, Fazekas A (2003) Biomaterials 24:4197
Acknowledgement
The authors wish to thank the Hungarian Research Foundation OTKA for scientific support (project numbers F042715 and M036688).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szekeres, M., Fodor, G., Fazekas, A. et al. Formation of octacalcium phosphate by heterogeneous nucleation on a titania surface. Colloid Polym Sci 283, 587–592 (2005). https://doi.org/10.1007/s00396-004-1188-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1188-y