Abstract
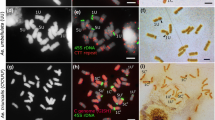
Roots fromAllium cepa L. (cv.Francesa) bulbs in which a maximum of two nucleoli per nucleus developed were selected for this study. Five rDNA clusters were detected by fluorescent in situ hybridization on chromosomal squashes (2n=16) with a rhodamine-labelled wheat, rDNA repeat. The rDNA clusters were located on four chromosomes: the largest cluster occurred on the small arm of a single homologue of the smallest pair 8. Its homologue showed two different small rDNA. clusters, one near each telomere. The two homologues of the satellited chromosomes 6 also showed different rDNA contents, which were intermediate to those found in pair 8. The same five well-differentiated hybridization signals were observed in interphase cells that were inactive in transcription because they were in dormant roots, or in proliferating ones in which the synthesis of the large rRNA precursor was prevented. After multipolarizing agent was applied in anaphase followed by inhibition of cytokinesis, multinucleate autotetraploid cells were formed, which often contained more than four nucleoli. Thus, at least two of the three nucleolar organizer regions that consistently failed to develop a nucleolus in normal mononucleate cells were capable of developing nucleoli when segregated into different nuclei in multinucleate cells.
Similar content being viewed by others
References
Bell SP, Learned RM, Jantzen HM, Tjian R (1988) Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 241: 1192–1197
Benavente R, Rose KM, Reimer R, Hügle-Dörr B, Scheer U (1987) Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol 105:1483–1491
Comai L, Zomerkijk J, Beckerman H, Zhou S, Admon A, Tjian R (1994) Reconstitution of transcription factor SL-1: exclusive binding of TBF by SL-1 or TFIID subunits. Science 266: 1966–1972
Cortés F, Escalza P (1986) Analysis of different banding patterns and late replicating regions in chromosomes ofAllium cepa, A. sativum andA. nigrum. Genetica 71:39–46
Cuadrado A, Jouve N (1994) Mapping and organization of highly-repeated DNA sequences by means of simultaneous and sequential FISH and C-banding in 6x-triticale. Chromosome Res 2:331–338
De la Torre C, Giménez-Abián JF, González-Fernández A (1991) Dominance of a NOR (nucleolar organizer region) over its allele and over its sister NOR after asymmetric 5-azacytidine substitution in plant chromosomes. J Cell Sci 100:667–674
Echols H (1990) Nucleoprotein structures initiating DNA replication, transcription and site-specific recombination. J Biol Chem 265:14697–14700
García-Blanco M, Miller DD, Sheetz MP (1995) Nuclear spreads: I. Visualization of bipartite ribosomal RNA domains. J Cell Biol 128:15–27
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Giménez-Abián MI, De la Torre C, López-Sáez JF, Cánovas JL (1989) Rates of cell entrance into different periods of the cell cycle sustain that negative exponential cell age distribution occurs inAllium cepa L. meristems. Cell Biol Int Rep 13: 845–850
Giménez-Martín G, González-Fernández A, De la Torre C, Fernández-Gómez ME (1971) Partial initiation of endomitosis by 3′-deoxyadenosine. Chromosoma 33:361–371
Giménez-Martín G, De la Torre C, Fernández-Gómez ME, González-Fernández A (1974) Experimental analysis of nucleolar reorganization. J Cell Biol 60:502–507
Giménez-Martín G, Panzera F, Cánovas JL, De la Torre C, López-Sáez JF (1992) A limited number of chromosomes makes a nucleus competent to respond to inducers of replication and mitosis in a plant. Eur J Cell Biol 58:163–171
Ingle J, Sinclair J (1972) Ribosomal RNA genes and plant development. Nature 235:30–32
Jiménez-García LF, Segura-Valdez ML, Ochs RL, Rothblum I, Hannan R, Spector DL (1994) Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell 5:955–966
Maggini F, Carmona MJ (1981) Sequence heterogeneity of the ribosomal DNA inAllium cepa (Liliaceae). Protoplasma 108: 163–171
Martini G, O'Dell M, Flavell RB (1982) Partial inactivation of wheat nucleolus organizer by the nucleolus organizer chromosomes fromAegilops umbellulata. Chromosoma 84:687–700
Morcillo G, De la Torre C (1979) Mapping nucleologenesis in relation to transcription. Biol Cell 36:1–6
Navashin M (1934) Chromosomal alterations caused by hybridization and their bearing upon certain general genetic problems. Cytologia 5:169–203
Penman S, Fan H, Perlman S, Rosbach M, Weinberg R, Zylber E (1970) Distinct RNA synthesis of the HeLa cell. Cold Spring Harbor Symp Quant Biol 35:561–575
Reeder RH (1985) Mechanisms of nucleolar dominance in animals and plants (a review). J Cell Biol 101:2013–2016
Ricroch A, Peffley EB, Baker RJ (1992) Chromosomal location of rDNA inAllium: in situ hybridization using biotin- and fluorescein-labelled probes. Theor Appl Genet 83:413–418
Roussel P, Hernández-Verdún D (1994) Identification of Ag-NOR proteins, markers of proliferation related to ribosomal gene activity. Exp Cell Res 214:465–472
Sato S (1981) Cytological studies on the satellited chromosomes ofAllium cepa. Caryologia 34:431–440
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions inAllium. Chromosoma 92: 143–148
Sparrow AH, Price HJ, Underbrink AG (1972) A survey of DNA content per cell and per chromosome of prokaryotic and eukaryotic organisms: some evolutionary considerations. Brookhaven Symp Biol 23:451–493
Wallace H, Eldredge WHR (1971) Differential amphiplasty and the control of ribosomal RNA synthesis. Heredity 27:1–13
Zomerdijk J, Beckmann H, Comai L, Tjian R (1994) Assembly of trancriptionally active RNA polymerase I initiation factor SL-1 from recombinant subunits. Science 266:2015–2018
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by: D. P. Bazett-Jones
Rights and permissions
About this article
Cite this article
Panzera, F., Giménez-Abián, M.I., López-Sáez, J.F. et al. Nucleolar organizer expression inAllium cepa L. chromosomes. Chromosoma 105, 12–19 (1996). https://doi.org/10.1007/BF02510034
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02510034