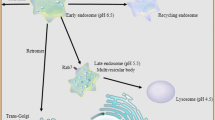
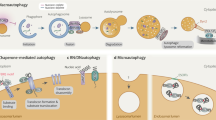
Abstract
Lysosomal enzymes are subjected to a number of modifications including carbohydrate restructuring and proteolytic maturation. Some of these reactions support lysosomal targeting, others are necessary for activation or keeping the enzyme inactive before being segregated, while still others may be adventitious. The non-segregated fraction of the enzyme is secreted and can be isolated from the medium. It is considered that the secreted lysosomal enzymes fulfill certain physiological and pathophysiological roles. By comparing the secreted and the intracellular enzymes it is possible to distinguish between the reactions that occur before and after the segregation. In this review the reactions that may influence the segregation are referred to as the early processing and those characteristic for the enzymes isolated from lysosomal compartments as the late processing. The early processing is characterized mainly by modifications of carbohydrate side chains. In the late processing, proteolytic fragmentation represents the most conspicuous changes. The review focuses on the compartmentation of the reactions and the proteolytic fragmentation of lysosomal enzyme precursors. While a plethora of proteolytic reactions are involved, our knowledge of the proteinases responsible for the particular maturation reactions remains very limited. The review points also to work with cells from patients affected with lysosomal storage disorders, which contributed to our understanding of the lysosomal apparatus.
Similar content being viewed by others
References
Abraham, D., Muir, H., Winchester, B., and Olsen, I., Lymphocytes transfer only the lysosomal form of α-mannosidase during cell-to-cell contract. Exp. Cell Res.175 (1988) 158–168.
Achord, D., Brot, F., Gonzalez-Noriega, A., Sly, W., and Stahl, P., Human β-glucuronidase. II. Fate of infused human placental β-glucuronidase in the rat. Pediat. Res.11 (1977) 816–822.
Achord, D. T., Brot, F. E., Bell, C. E., and Sly, W. S., Human β-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell15 (1978) 269–278.
Achstetter, T., Franzusoff, A., Field, C., and Schekman, R., SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J. biol. Chem.263 (1988) 11711–11717.
Aerts, J. M. F. G., Schram, A. W., Strijland, A., van Weely, S., Jonsson, L. M. V., Tager, J. M., Sorrell, S. H., Ginns, E. I., Barranger, J. A., and Murray, G. J., Glucocerebrosidase, a lysosomal enzyme that does not undergo oligosaccharide phosphorylation. Biochim. biophys. Acta964 (1988) 303–308.
Akin, D. T., and Kinkade, J. M. Jr, Processing of a newly identified intermediate of human myeloperoxidase in isolated granules occurs at neutral pH. J. biol. Chem.261 (1986) 8370–8375.
Akin, D. T., and Kinkade, J. M. Jr, Evidence for the involvement of an acidic compartment in the processing of myeloperoxidase in human promyelocytic leukemia HL-60 cells. Archs Biochem. Biophys.255 (1987) 428–436.
Ammerer, G., Hunter, C. P., Rothman, J. H., Saari, G. C., Valls, L. A., and Stevens, T. H., PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Molec. cell. Biol.6 (1986) 2490–2499.
Argade, S. P., Hopfer, R. L., Strang, A-M., van Halbeek, H., and Alhadeff, J. A., Structural studies on the carbohydrate moieties of human liver α-L-fucosidase. Archs Biochem. Biophys.266 (1988) 227–247.
Ashwell, G., and Morell, H. G., The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv. Enzymol.41 (1974) 99–128.
Assfalg-Machleidt, I., Jochum, M., Klaubert, W., Inthorn, D., and Machleidt, W., Enzymatically active cathepsin B dissociating from its inhibitor complexes is elevated in blood plasma of patients with septic shock and some malignant tumors. Biol. Chem. Hoppe-Seyler369 (1988) suppl. 263–269.
Astrin, K. H., and Desnick, R. J., Chromosomal localization of the structural genes encoding the human lysosomal hydrolases and their activator and stabilizer proteins, in: Molecular Basis of Lysosomal Storage Disorders, pp. 325–363. Eds J. A. Barranger and R. O. Brady. Academic Press, Inc., Orlando 1984.
Bainton, D. F., The discovery of lysosomes. J. Cell Biol.91 (1981) 66s-76s.
Bainton, D. F., and Farquhar, M. G., Origin of granules in polymorphonuclear leukocytes. J. Cell Biol.28 (1966) 277–301.
Bainton, D. F., and Farquhar, M. G., Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. J. Cell Biol.39 (1968) 286–298.
Bainton, D. F., Ullyot, J. L., and Farquhar, M. G., The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. exp. Med.134 (1971) 907–934.
Ballabio, A., Parenti, G., Carrozzo, R., Sebastio, G., Andria, G., Buckle, V., Fraser, N., Craig, I., Rocchi, M., Romeo, G., Jobsis, A. C., and Persico, M. G., Isolation and characterization of a steroid sulfatase cDNA clone: genomic deletions in patients with X-chromosome-linked ichthyosis. Proc. natl Acad. Sci. USA84 (1987) 4519–4523.
Baranski, T. J., Faust, P. L., and Kornfeld, S., Generation of a lysosomal enzyme targeting signal in the secretory protein pepsinogen. Cell63 (1990) 281–291.
Barrett, A. J., Human cathepsin D. Adv. exp. Med. Biol.95 (1979) 291–300.
Barrett, A. J., Buttle, D. J., Mason, R. W., Lysosomal Cysteine Proteinases. ISI Atlas of Sci. Biochem. (1988) 256–260.
Barriocanal, J. G., Bonifacino, J. S., Yuan, L., and Sandoval, I. V., Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J. biol. Chem.261 (1986) 16755–16763.
Barton, K. A., Thompson, J. F., Madison, J. T., Rosenthal, R., Jarvis, N. P., and Beachy, R. N., The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J. biol. Chem.257 (1982) 6089–6095.
Bielicki, J., Freeman, C., Clements, P. R., and Hopwood, J. J., Human liver iduronate-2-sulphatase. Biochem. J.271 (1990) 75–86.
Bishop, D. F., Calhoun, D. H., Bernstein, H. S., Hantzopoulos, P., Quinn, M., and Desnick, R. J., Human α-galactosidase A: Nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc. natl Acad. Sci. USA83 (1986) 4859–4863.
Blum, J. S., Diaz, R., Diment, S., Fiani, M., Mayorga, L., Rodman, J. S., and Stahl, P. D., Proteolytic processing in endosomal vesicles. Cold Spring Harbor Symp. quant. Biol.54 (1989) 287–292.
Bonifas, J. M., Morley, B. J., Oakey, R. E., Kan, Y. W., and Epstein, Jr, E. H., Cloning of a cDNA for steroid sulfatase: frequent occurrence of gene deletions in patients with recessive X chromosome-linked ichthyosis. Proc. natl Acad. Sci. USA84 (1987) 9248–9251.
Braulke, T., Hasilik, A., and von Figura, K., Low temperature blocks transport and sorting of cathepsin D in fibroblasts. Biol. Chem. Hoppe-Seyler369 (1988) 441–449.
Braulke, T., Hille, A., Huttner, W. B., Hasilik, A., and von Figura, K., Sulfated oligosaccharides in human lysosomal enzymes. Biochem. biophys. Res. Commun.143 (1987) 178–185.
Braun, M., Waheed, A., and von Figura, K., Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J.8 (1989) 3633–3640.
Brown, J. A., Jahreis, G. P., and Swank, R. T., The synthesis and processing of β-glucuronidase in normal and egasyn deficient mouse kidney. Biochem. biophys. Res. Commun.99 (1981) 691–699.
Brown, J. A., and Swank, R. T., Subcellular redistribution of newly synthesized macrophage lysosomal enzymes. J. biol. Chem.258 (1983) 15323–15328.
Burg, J., Banerjee, A., and Sandhoff, K., Molecular forms of GM2-activator protein. Biol. Chem. Hoppe-Seyler366 (1985) 887–891.
Burkhardt, J. K., Hester, S., Lapham, C. K., and Argon, Y., The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J. Cell Biol.111 (1990) 2327–2340.
Bush, J. M., and Cardelli, J. A., Processing, transport, and secretion of the lysosomal enzyme acid phosphatase in Dictyostelium discoideum. J. biol. Chem.264 (1989) 7630–7636.
Byrd, J. C., and Touster, O., Isolation and partial characterization of the oligosaccharide moieties of rat preputial β-glucuronidase. Biochim. biophys. Acta677 (1981) 69–78.
Cardelli, J. A., Bush, J. M., Ebert, D., and Freeze, H. H., Sulfated N-linked oligosaccharides affect secretion but are not essential for the transport, proteolytic processing, and sorting of lysosomal enzymes in Dictyostelium discoideum. J. biol. Chem.265 (1990) 8847–8853.
Cardelli, J. A., Golumbeski, G. S., and Dimond, R. L., Lysosomal enzymes in Dictyostelium discoideum are transported to lysosomes at distinctly different rates. J. Cell Biol.102 (1986) 1264–1270.
Carlsson, S. R., Roth, J., Piller, F., and Fukuda, M., Isolation and characterization of human lysosomal membrane glycoproteins, hlamp-1 and h-lamp-2. J. biol. Chem.263 (1988) 18911–18919.
Carver, J.PP., and Brisson, J.-R., The three-dimensional structure of N-linked oligosaccharides, in: Biology of Carbohydrates, vol. 2, pp. 289–331. Eds V. Ginsburg and P. W. Robbins. John Wiley & Sons, New York 1984.
Chan, S. J., San Segundo, B., McCormick, M. B., and Steiner, D. F., Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc. natl Acad. Sci. USA83 (1986) 7721–7725.
Ching, I-L., and Dice, J. F., Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J. biol. Chem.263 (1988) 6797–6805.
Chiang, H-L., and Schekman, R., Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature350 (1991) 313–318.
Chiang, H-L., Terlecky, S. R., Plant, C. P., and Dice, J. F., A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science246 (1989) 382–385.
Chrispeels, M. J., Higgins, T. J. V., Craig, S., and Spencer, D., Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J. Cell Biol.93 (1982) 5–14.
Chrispeels, M. J., Higgins, T. J. V., and Spencer, D., Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J. Cell Biol.93 (1982) 306–313.
Cohn, Z. A., and Fedorko, M. E., The formation and fate of lysomes, in: Lysosomes in Biology and Pathology, vol. 1, pp. 43–63. Eds J. T. Dingle and H. B. Fell, North Holland Publishing Company, Amsterdam 1969.
Collard, M. W., Sylvester, S. R., Tsurula, J. K., and Griswold, M. D., Biosynthesis and molecular cloning of sulfated glycoprotein 1 secreted by rat sertoli cells: sequence similarity with the 70-kilodalton precursor to sulfatide/GM1 activator. Biochemistry27 (1988) 4557–4564.
Conner, G. E., Isolation of procathepsin D from nature cathepsin D by pepstatin affinity chromatography. Biochem. J.263 (1989) 601–604.
Conner, G. E., The role of the cathepsin D propeptide in folding and in sorting to the lysosome. (1992) in press,
Conner, G. E., Udey, J. A., Pinto, C., and Sola, J., Nonhuman cells correctly sort and process the human lysosomal enzyme cathepsin D. Biochemistry28 (1989) 3530–3533.
Creek, L. E., and Sly, W. S., The role of the phosphomannosyl receptor in the transport of acid hydrolases to lysosomes, in: Lysosomes in Biology and Pathology, pp. 63–82. Eds J. T. Dingle, R. T. Dean and W. Sly, Elsevier Sci. Publ., Amsterdam 1984.
Croy, R. R. D., Lycett, G. W., Gatehouse, J. A., Yarwood, J. N., and Boulter, D., Cloning and analysis of cDNAs encoding plant storage protein precursors. Nature295 (1982) 76–79.
Dahms, M. M., Lobel, P., and Kornfeld, S., Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. biol. Chem.264 (1989) 12115–12118.
Davidson, H. W. and Watts, C., Epitope-directed processing of specific antigen by B lymphocytes. J. Cell Biol.109 (1989) 85–92.
d'Azzo, A., Hoogeveen, A., Reuser, A. J. J., Robinson, D., and Galjaard, H., Molecular defect in combined β-galactosidase and neuraminidase deficiency in man. Proc. natl Acad. Sci. USA79 (1982) 4535–4539.
d'Azzo, A., Proia, R. L., Kolodny, E. H., Kaback, M. M., and Neufeld, E. F., Faulty association of α- and β-subunits in some forms of β-hexosaminidase A deficiency. J. biol. Chem.259 (1984) 11070–11074.
de Duve, C., Lysosomes revisited. Eur. J. Biochem.137 (1983) 391–397.
de Duve, C., Gianetto, R., Appelmans, F., Wattiaux, R., Enzymic content of the mitochondria fraction. Nature172 (1953) 1143–1144.
Deshaies, R. J., Kepes, F., and Bohni, P. C., Genetic dissection of the early stages of protein secretion in yeast. Trends Genet.5 (1989) 87–93.
Dewji, N. N., Wenger, D. A., and O'Brien, J. S., Nucleotide sequence of cloned cDNA for human sphingolipid activator protein 1 precursor. Proc. natl Acad. Sci. USA84 (1987) 8652–8656.
Dice, J. F., Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends biochem. Sci.15 (1990) 305–309.
DiCioccio, R. A., Darby, J. K., and Willems, P. J., Abnormal expression of alpha-L-fucosidase in lymphoid cell lines of fucosidosis patients. Biochem. Genet.27 (1989) 279–290.
Diment, S., Leech, M. S., and Stahl, P. D., Cathepsin D is membrane-associated in macrophage endosomes. J. biol. Chem.263 (1988) 6901–6907.
Diment, S., and Stahl, P., Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J. biol. Chem.260 (1985) 15311–15317.
Distel, B., Al, E. J. M., Tabak, H. F., and Jones, E. W., Synthesis and maturation of the yeast vacuolar enzymes carboxypeptidase Y and aminopeptidase I. Biochim. biophys. Acta741 (1983) 128–135.
Dlott, B., d'Azzo, A., Quon, D. V. K., and Neufeld, E. F., Two mutations produce intron insertion in mRNA and elongated β-subunit of human β-hexosaminidase. J. biol. Chem.265 (1990) 17921–17927.
Do, K-Y., Smith, D. F., and Cummings, R. D., Lamp-1 in CHO cells is a primary carrier of poly-N-acetyllactosamine chains and is bound preferentially by a mammalian S-type lectin. Biochem. biophys. Res. Commun.173 (1990) 1123–1128.
Docherty, K., Carroll, R., and Steiner, D. F., Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc. natl. Acad. Sci. USA80 (1983) 3245–3249.
Docherty, K., Hutton, J. C., and Steiner, D. F., Cathepsin B-related proteases in the insulin secretory granule. J. biol. Chem.259 (1984) 6041–6044.
Domoney, C., and Casey, R., Storage protein precursor polypeptides in cotyledons ofPisum sativum L. Eur. J. Biochem.139 (1984) 321–327.
Dunn, W. A. Jr, Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J. Cell Biol.110 (1990) 1935–1945.
Elliott, T., Townsend, A., and Cerundolo, V., Naturally processed peptides. Nature348 (1990) 195–196.
Erickson, A. H., Biosynthesis of lysosomal endopeptidases. J. cell. Biochem.40 (1989) 31–41.
Erickson, A. H., and Blobel, G., Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J. biol. Chem.254 (1979) 11771–11774.
Erickson, A. H., and Blobel, G., Carboxy-terminal proteolytic processing during biosynthesis of the lysosomal enzymes β-glucuronidase and cathepsin D. Biochemistry22 (1983) 5201–5205.
Erickson, A. H., Conner, G. E., and Blobel, G., Biosynthesis of a lysosomal enzyme. J. biol. Chem.256 (1981) 11224–11231.
Erickson, A. H., Ginns, E. I., and Barranger, G., Biosynthesis of β-glucocerebrosidase in human fibroblasts. Fedn Proc.44 (1985) 709a.
Erickson, A. H., Ginns, E. I., and Barranger, J. A., Biosynthesis of the lysosomal enzyme glucocerebrosidase. J. biol. Chem.260 (1985) 14319–14324.
Erickson, A. H., Walter, P., and Blobel, G., Translocation of a lysosomal enzyme across the microsomal membrane requires signal recognition particle. Biochem. biophys. Res. Commun.115 (1983) 275–280.
Farquhar, M. G., and Palade, G. E., The Golgi apparatus (complex) (1954–1981)-from artifact to center stage. J. Cell Biol.91 (1981) 77s–103s.
Faust, P. L., Kornfeld, S., and Chirgwin, J. M., Cloning and sequence analysis of cDNA for human cathepsin D. Proc. natl Acad. Sci. USA82 (1985) 4910–4914.
Febbraio, M., and Silverstein, R. L., Identification and characterization of lamp-1 as an activation-dependent platelet surface glycoprotein. J. biol. Chem.265 (1990) 18531–18537.
Felleisen, R., and Klinkert, M-Q., In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J.9 (1990) 371–377.
Fisher, K. I., and Aronson, N. N. Jr, Isolation and sequence analysis of a cDNA encoding rat liver α-L-fucosidase. Biochem. J.264 (1989) 695–701.
Foltmann, B., Structure and function of proparts in zymogens for aspartic proteinases. Biol. Chem. Hoppe-Seyler369 (1988) suppl. 311–314.
Fong, D., Calhoun, D. H., Hsieh, W-T., Lee, B., and Wells, R. D., Isolation of cDNA clone for the human lysosomal proteinase cathepsin B. Proc. natl Acad. Sci. USA83 (1986) 2909–2913.
Frisch, A., Baram, D., and Navon, R., Hexosaminidase A deficient adults: Presence of α chain precursor in cultured skin fibroblasts. Biochem. biophys. Res. Commun.119 (1984) 101–107.
Frisch, A., and Neufeld, E. F., Limited proteolysis of the β-hexosaminidase precursor in a cell-free system. J. biol. Chem.256 (1981) 8242–8246.
Fuchs, R., and Gassen, H. G., Nucleotide sequence of human preprocathepsin H, a lysosomal cysteine proteinase. Nucl. Acids Res.17 (1989) 9471 only.
Fuchs, R., Machleidt, W., and Gassen, H. G., Molecular cloning and sequencing of a cDNA coding for mature human kidney cathepsin H. Biol. Chem. Hoppe-Seyler369 (1988) 469–475.
Fürst, W., Schubert, J., Machleidt, W., Meyer, H. E., and Sandhoff, K., The complete amino-acid sequences of human ganglioside GM2 activator protein and cerebroside sulfate activator protein. Eur. J. Biochem.192 (1990) 709–714.
Fürst, W., Machleidt, W., and Sandhoff, K., The precursor of sulfatide activator protein is processed to three different proteins. Biol. Chem. Hoppe-Seyler369 (1988) 317–328.
Fujibayashi, S., and Wenger, D. A., Synthesis and processiing of sphingolipid activator protein-2 (SAP-2) in cultured human fibroblasts. J. biol. Chem.261 (1986) 15339–15343.
Fukuda, M., Viitala, J., Matteson, J., and Carlsson, S. R., Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. J. biol. Chem.263 (1988) 18920–18928.
Fukushima, H., de Wet, J. R., and O'Brien, J. S., Molecular cloning of a cDNA for human α-L-fucosidase. Proc. natl Acad. Sci. USA82 (1985) 1262–1265.
Furuno, K., Yano, S., Akasaki, K., Tanaka, Y., Yamaguchi, Y., Tsuji, H., Himeno, M., and Kato, R., Biochemical analysis of the movement of a major lysosomal membrane glycoprotein in the endocytic membrane system. J. Biochem. Tokyo106 (1989) 717–722.
Gabay, J. E., Heiple, J. M., Cohn, Z. Y., and Nathan, C. F., Subcellular location and properties of bactericidal factors from human neutrophils. J. exp. Med.164 (1986) 1407–1421.
Gabel, C. A., and Kornfeld, S., Targeting of β-glucuronidase to lysosomes in mannose 6-phosphate receptor-deficient MOPC 315 cells. J. Cell Biol.99 (1984) 296–305.
Gal, S., and Gottsman, M. M., The major excreted protein of transformed fibroblasts in an activable acid-protease. J. biol. Chem.261 (1986) 1760–1765.
Gal, S., Willingham, M. C., and Gottesman, M. M., Processing and lysosomal localization of a glycoprotein whose secretion is transformed stimulated. J. Cell Biol.100 (1985) 535–544.
Galjart, N. J., Gillemans, N., Harris, A., van der Horst, G. T. J., Verheijen, F. W., Galjaard, H., and d'Azzo, A., Expression of cDNA encoding the human “protective protein” associated with lysosomal β-galactosidase and neuraminidase: homology to yeast proteases. Cell54 (1988) 755–764.
Galjart, N. J., Gillemans, N., Meijer, D., and d'Azzo, A., Mouse “protective protein”. J. biol. Chem.265 (1990) 4678–4684.
Gatehouse, J. A., Croy, R. R. D., Morton, H., Tyler, M., and Boulter, D., Characterisation and subunit structures of the vicillin storage proteins of pea (Pisum sativum L.). Eur. J. Biochem.118 (1981) 627–633.
Gatehouse, J. A., Lycett, G. W., Croy, R. R. D., and Boulter, D., The post-translational proteolysis of the subunits of vicilin from pea (Pisum sativum L.). Biochem. J.207 (1982) 629–632.
Gierasch, L. M., Signal sequences, Biochemistry28 (1989) 923–930.
Gieselmann, V., Hasilik, A., and von Figura, K., Processing of human cathepsin D in lysosomes in vitro. J. biol. Chem.260 (1985) 3215–3220.
Gieselmann, V., Pohlmann, R., Hasilik, A., and von Figura, K., Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J. Cell Biol.97 (1983) 1–5.
Gieselmann, V., Polten, A., Kreysing, J., and von Figura, K., Arylsulfatase A pseudodeficiency: loss of a polyadenylylation signal and N-glycosylation site. Proc. natl Acad. Sci. USA86 (1989) 9436–9440.
Glauman, H., and Ballard, F. J., (Eds), Lysosomes: Their Role in Protein Breakdown, pp. 1–737. Academic Press, London 1987.
Goldberg, D., Gabel, C., and Kornfeld, S., Processing of lysosomal enzyme oligosaccharide units, in: Lysosomes in Biology and Pathology, pp. 45–62. Eds J. T. Dingle, R. T. Dean and W. Sly. Elsevier Sci. Publ. Amsterdam 1984.
Goldstein, J. L., and Brown, M. S., The low density lipoprotein pathway and its relation to atherosclerosis. A. Rev. Biochem.46 (1977) 897–930.
Gonzalez-Noriega, A., Grubb, J. H., Talkad, V., and Sly, W. S., Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J. Cell Biol.85 (1980) 839–852.
Gordon, P. B., and Seglen, P. O., Prelysosomal convergence of autophagic and endocytic pathways. Biochem. biophys. Res. Commun.151 (1988) 40–47.
Gottschalk, S., Waheed, A., and von Figura, K., Targeting of lysosomal acid phosphatase with altered carbohydrate. Biol. Chem. Hoppe-Seyler370 (1989) 75–80.
Gottschalk, S., Waheed, A., Schmidt, b., Laidler, P., and von Figura, K., Sequential processing of lysosomal acid phosphatase by a cytoplasmic thiol proteinase and a lysosomal aspartyl proteinase. EMBO J.8 (1989) 3215–3219.
Green, S. A., Zimmer, K-P., Griffiths, G., and Mellman, I., Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol.105 (1987) 1227–1240.
Griffiths, G., Hoflack, B., Simons, K., Mellman, I., and Kornfeld, S., The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell52 (1988) 329–341.
Guagliardi, L. E., Koppelman, B., Blum, J. S., Marks, M. S., Cresswell, P., and Brodsky, F. M., Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature343 (1990) 133–139.
Gupta, D. K., Schmidt, A., von Figura, K., and Hasilik, A., Processing and transport of lysosomal enzymes in human monocyte line U937. Hoppe-Seyler's Z. Physiol. Chem.365 (1984) 867–876.
Haltiwanger, R. S., and Hill, R. L., The ligand binding specificity and tissue localization of a rat alveolar macrophage lectin. J. biol. Chem.261 (1986) 15696–15702.
Hancock, L. W., Ricketts, J. P., and Hildreth, IV, J., Impaired proteolytic processing of lysosomal N-acetyl-β-hexosaminidase in cultured fibroblasts from patients with infantile generalized N-acetylneuraminic acid storage disease. Biochem. biophys. Res. Commun.152 (1988) 83–92.
Hanewinkel, H., Glössl, J., and Kresse, H., Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J. biol. Chem.262 (1987) 12351–12355.
Hara, K., Kominami, E., and Katunuma, N., Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett.231 (1988) 229–231.
Harding, C. V., Collins, D. S., Slot, J. W., Geuze, H. J., and Unanue, E. R., Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell64 (1991) 393–401.
harding, C. V., and Unanue, E. R., Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J. Immun.142 (1989) 12–19.
Hasilik, A., and Neufeld, E. F., Biosynthesis of lysosomal enzymes in fibroblasts. J. biol. Chem.255 (1980) 4937–4945.
Hasilik, A., and Neufeld, E. F., Biosynthesis of lysosomal enzymes in fibroblasts. J. biol. Chem.255 (1980) 4946–4950.
Hasilik, A., Pohlmann, R., and von Figura, K., Inhibition by cyanate of the processing of lysosomal enzymes. Biochem. J.210 (1983) 795–802.
Hasilik, A., Pohlmann, R., Olsen, R. L., and von Figura, K., Myeloperoxidase is synthesized as larger phosphorylated precursor. EMBO J.3 (1984) 2671–2676.
Hasilik, A., and Tanner, W., Biosynthesis of carboxypeptidase Y in yeast. Biochem. biophys. Res. Commun.72 (1976) 1430–1436.
Hasilik, A., and Tanner, W., Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Eur. J. Biochem.85 (1978) 599–608.
Hasilik, A., and von Figura, K., Oligosaccharides in lysosomal enzymes. Eur. J. Biochem.121 (1981) 125–129.
Hasilik, A., and von Figura, K., Processing of lysosomal enzymes in fibroblasts, in: Lysosomes in Biology and Pathology, pp. 3–16. Eds J. T. Dingle, R. T. Dean and W. Sly. Elsevier Sci. Publ., Amsterdam 1984.
Hasilik, A., and von Figura, K., Lysosomal enzymes and the Golgi apparatus, in: Cellular and Molecular Events in Spermiogenesis, pp. 59–75. Eds D. W. Hamilton and G. M. H. Waites. Cambridge University Press, Cambridge 1990.
Hasilik, A., von Figura, K., Conzelmann, E., Nehrkorn, H., and Sandhoff, K., Lysosomal enzyme precursors in human fibroblasts. Eur. J. Biochem.125 (1982) 317–321.
Hattori, T., Ichihara, S., and Nakamura, K., Processing of a plant vacuolar protein precursor in vitro. Eur. J. Biochem.166 (1987) 533–538.
Hemmings, B. A., Zubenko, G. S., Hasilik, A., and Jones, E. W., Mutant defective in processing of an enzyme located in the lysosomelike vacuole of Saccharomyces cerevisiae. Proc. natl Acad. Sci. USA78 (1981) 435–439.
Hentze, M., Hasilik, A., and von Figura, K., Enhanced degradation of cathepsin D synthesized in the presence of the threonine analog β-hydroxynorvaline. Archs Biochem. Biophys.230 (1984) 375–382.
Herman, E. M., Baumgartner, B., and Chrispeels, M. J., Uptake and apparent digestion of cytoplasmic organelles by protein bodies (protein storage vacuoles) in mung bean cotyledons. Eur. J. Cell Biol.24 (1981) 226–235.
Himeno, M., Fujita, H., Noguchi, Y., Kono, A., and Kato, K., Isolation and sequencing of a cDNA clone encoding acid phosphatase in rat liver lysosomes. Biochem. biophys. Res. Commun.162 (1989) 1044–1053.
Hineno, T., Sano, A., Kondoh, K., Ueno, S-i., Kakimoto, Y., and Yoshida, K-i., Secretion of sphingolipid hydrolase activator precursor, prosaposin. Biochem. biophys. Res. Commun.176 (1991) 668–674.
Hoefsloot, L. H., Hoogeveen-Westerveld, M., Kroos, M. A., van Beeumen, J., Reuser, A. J. J., and Oostra, B. A., Primary structure and processing of lysosomal α-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J.7 (1988) 1697–1704.
Holmsen, H., and Dangelmeier, C. A., Measurement of secretion of lysosomal acid glycosidases. Meth. Enzymol.169 (1989) 336–342.
Holtzman, E., Lysosomes, pp. 1–439. Plenum Press, New York 1989.
Holzer, H., and Heinrich, P. C., Control of proteolysis. A. Rev. Biochem.49 (1980) 63–91.
Hoogeween, A. T., Graham-Kawashima, H., d'Azzo, A., and Galjaard, H., Processing of human β-galactosidase in GM1-gangliosidosis and Morquio B syndrome. J. biol. Chem.259 (1984) 1974–1977.
Horst, M., and Hasilik, A., Expression and maturation of human cathepsin D in baby-hamster kidney cells. Biochem. J.273 (1991) 355–361.
Howard, D. R., Natowicz, M., and Baenziger, J. U., Structural studies of the endoglycosidase H-resistant oligosaccharides present on human β-glucuronidase. J. biol. Chem.257 (1982) 10861–10868.
Hu, P., Reuser, A. J. J., Janse, H. C., Kleijer, W. J., Schindler, D., Sakuraba, H., Tsuji, A., Suzuki, Y., and van Diggelen, O. P., Biosynthesis of human α-N-acetylgalactosaminidase: defective phosphorylation and maturation in infantile α-NAGA deficiency. Biochem. biophys. Res. Commun.175 (1991) 1097–1103.
Hubbes, M., Callahan, J., Gravel, R., and Mahuran, D., The aminoterminal sequences in the pro-α and-β polypeptides of human lysosomal β-hexosaminidase A and B are retained in the mature isozymes. FEBS Lett.249 (1989) 316–320.
Hünseler, P., Tiedtke, A., and von Figura, K., Biosynthesis of secreted β-hexosaminidase in Tetrahymena thermophila. Biochem. J.252 (1988) 837–842.
Imai, K., Characterization of β-glucosidase as a peripheral enzyme of lysosomal membranes from mouse liver and purification. J. Biochem., Tokyo98 (1985) 1405–1416.
Imort, M., Zühlsdorf, M., Feige, U., Hasilik, A., and von Figura, K., Biosynthesis and transport of lysosomal enzymes in human monocytes and macrophages. Biochem. J.214 (1983) 671–678.
Isaksson, A., and Hultberg, B., Immunoassay of betahexosaminidase isoenzymes in serum in patients with raised total activities. Clinica chim. Acta183 (1989) 155–162.
Ishidoh, K., Kominami, E., Katunuma, N., and Suzuki, K., Gene structure of rat cathepsin H. FEBS Lett.253 (1989) 103–107.
Ishidoh, K., Kominami, E., Suzuki, K., and Katunuma, N., Gene structure and 5′-upstream sequence of rat cathepsin L. FEBS Lett.259 (1989) 71–74.
Ishidoh, K., Towatari, T., Imajoh, S., Kawasaki, H., Kominami, E., Katunuma, N., and Suzuki, K., Molecular cloning and sequencing of cDNA for rat cathepsin L. FEBS Lett.223 (1987) 69–73.
Isidoro, C., Horst, M., Baccino, F. M., and Hasilik, A., Differential segregation of human and hamster cathepsin D in transfected baby-hamster kidney cells. Biochem. J.273 (1991) 363–367.
Iwamoto, H., Morita, Y., Kobayashi, T., Hasegawa, E., Subunit structures of three human myeloperoxidases. J. Biochem., Tokyo103 (1988) 688–692.
Jackman, H. L., Tan, F., Tamei, H., Beurling-Harbury, C., Li, X-Y., Skidgel, R. A., and Erdös, E. G., A peptidase in human platelets that deamidates tachykinis. J. biol. Chem.265 (1990) 11265–11272.
Johnson, K., and Dawson, G., Molecular defect in processing α-fucosidase in fucosidosis. Biochem. biophys. Res. Commun.133 (1985) 90–97.
Johnson, E. D., Knight, J., and Gayler, K. R., Biosynthesis and processing of legumin-like storage proteins inLupinus angustifolius (lupin). Biochem. J.232 (1985) 673–679.
Jonsson, L. M. V., Murray, G. J., Sorrell, S. H., Strijland, A., Aerts, F. F. G. M., Ginns, E. I., Barranger, J. A., Tager, J. M., and Schram, A. W., Biosynthesis and maturation of glucocerebrosidase in Gaucher fibroblasts. Eur. J. Biochem.164 (1987) 171–179.
Joseph, L. J., Chang, L. C., Stamenkovich, D., and Sukhatme, V. P., Complete nucleotide and deduced amino acid sequences of human and murine preprocathepsin L. J. clin. Invest.81 (1988) 1621–1629.
Jung, K., Diego, J., Strobelt, V., Scholz, D., and Schreiber, G., Diagnostic Significance of some urinary enzymes for detecting acute rejection crises in renal-transplant recipients: alanine aminopeptidase, alkaline phosphatase, τ-glutamyltransferase, N-acetyl-β-D-glucosaminidase, and lysozyme. Clin. Chem.32 (1986) 1807–1811.
Kageyama, T., and Takahashi, K., Activation mechanism of monkey and porcine pepsinogens A. Eur. J. Biochem.165 (1987) 483–490.
Kaneko, Y., Hayashi, N., Toh-e, A., Banno, I., and Oshima, Y., Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase inSaccharomyces cerevisiae. Gene58 (1987) 137–148.
Keppler, D., Pagano, M., Dalet-Fumeron, V., and Engler, R., Purification and characterization of two different precursor forms of the cathepsin B-like proteinase from human malignant ascitic fluid. Biol. Chem. Hoppe-Seyler369 (1988) suppl. 185–190.
Klebanoff, S. J., and Clark, R. A., The Neutrophil: Function and Clinical Disorders, pp. 1–810. North-Holland Publishing Company, Amsterdam 1978.
Kleinschmidt, T., Christomanou, H., and Braunitzer, G., Complete amino-acid sequence and carbohydrate content of the naturally occurring glucosylceramide activator protein (A1 activator) absent from a new human Gaucher disease variant. Biol. Chem. Hoppe-Seyler368 (1987) 1571–1578.
Klionsky, D. J., Banta, L. M., and Emr, S. D., Intracellular sorting and processing of a yeast vacuolar hydrolase: Proteinase A propeptide contains vacuolar targeting information. Molec. cell. Biol.8 (1988) 2105–2116.
Klionsky, D. J., and Emr, S. D., Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J.8 (1989) 2241–2250.
Klionsky, D. J., and Emr, S. D., A new class of lysosomal/vacuolar protein sorting signals. J. biol. Chem.265 (1990) 5349–5352.
Klionsky, D. J., Herman, P. K., and Emr, S. D., The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev.54 (1990) 266–292.
Koeffler, H. P., Ranyard, J., and Pertcheck, M., Myeloperoxidase: its structure and expression during myeloid differentiation. Blood65 (1985) 484–491.
Kominami, E., and Katunuma, N., Biosyntheses, processings and localizations of lysosomal cysteine proteinases, in: Intracellular Proteolysis, Mechanisms and Regulations, pp. 52–60. Eds N. Katunuma and E. Kominami. Japan Scientific Societies Press, Tokyo 1989.
Kominami, E., Tsukahara, T., Bando, Y., and Katunuma, N., Autodegradation of lysosomal cysteine proteinases. Biochem. biophys. Res. Commun.144 (1987) 749–756.
Kominami, E., Tsukahara, T., Hara, K., and Katunuma, N., Biosyntheses and processing of lysosomal cysteine proteinases in rat macrophages. FEBS Lett.231 (1988) 225–228.
Kopitz, J., Kiesen, G. Ö., Gordon, P. B., Bohley, P., and Seglen, P. O., Nonselective autophagy of cytosolic enzymes by isolated rat hepatocytes. J. Cell Biol.111 (1990) 941–953.
Korchak, H. M., Vienne, K., Rutherford, L. E., and Weissmann, G., Neutrophil stimulation: receptor, membrane, and metabolic events. Fedn Proc.43 (1984) 2749–2754.
Korneluk, R. G., Mahuran, D. J., Neote, K., Klavins, M. H., O'Dowd, B. F., Tropak, M., Willard, H. F., Anderson, M-J., Lowden, J. A., and Gravel, R. A., Isolation of cDNA clones coding for the α-subunit of human β-hexosaminidase. J. biol. Chem.261 (1986) 8407–8413.
Kornfeld, S., Trafficking of lysosomal enzymes in normal and disease states. J. clin. Invest.77 (1986) 1–6.
Kornfeld, S., Trafficking of lysosomal enzymes. FASEB J.1 (1987) 462–468.
Kornfeld, S., and Mellman, I., The biogenesis of lysosomes. A. Rev. Cell Biol.5 (1989) 483–525.
Kornreich, R., Desnick, R. J., and Bishop, D. F., Nucleotide sequence of the human α-galactosidase A gene. Nucl. Acids Res.17 (1989) 3301–3302.
Krentler, C., Pohlmann, R., Hasilik, A., and von Figura, K., Lysosomal membrane proteins do not bind to mannose 6-phosphate-specific recepters. Biol. Chem. Hoppe-Seyler367 (1986) 141–145.
Laidler, P. M., Waheed, A., and Van Etten, R. L., Structural and immunochemical characterization of human urine arylsulfatase A purified by affinity chromatography. Biochim. biophys. Acta827 (1935) 73–83.
Lau, M. M. H., and Neufeld, E. F., A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the α-subunit of β-hexosaminidase. J. biol. Chem.264 (1989) 21376–21380.
Lehrer, R. I., and Ganz, T., Antimicrobial polypeptides of human neutrophils. Blood76 (1990) 2169–2181.
Leibold, D. M., Robinson, C. B., Scanlin T. F., and Glick, M. C., Lack of proteolytic processing of α-L-fucosidase in human skin fibroblasts. J. Cell Physiol.137 (1988) 411–420.
Lennartz, M. R., Cole, F. S., Shepherd, V. L., Wileman, T. E., and Stahl, P. D., Isolation and characterization of a mannose-specific endocytosis receptor from human placenta. J. biol. Chem.262 (1987) 9942–9944.
Lennartz, M. R., Wileman, T. E., and Stahl, P. D., Isolation and characterization of a mannose-specific endocytosis receptor from rabbit alveolar macrophages. Biochem. J.245 (1987) 705–711.
Lippincott-Schwartz, J., and Fambrough, D. M., Lysosomal membrane dynamics: Structure and interorganellar movement of a major lysosomal membrane glycoprotein. J. Cell Biol.192 (1986) 1593–1605.
Little, L. E., Lau, M. M. H., Quon, D. V. K., Fowler, A. V., and Neufeld, E. F., Proteolytic processing of the α-chain of the lysosomal enzyme, β-hexosaminidase, in normal human fibroblasts. J. biol. Chem.263 (1988) 4288–4292.
Lord, J. M., Precursors of ricin andRicinus communis agglutinin. Eur. J. Biochem.146 (1985) 411–416.
Mahuran, D. J., Neote, K., Klavins, M. H., Leung, A., and Gravel, R. A., Proteolytic processing of pro-α and pro-β precursors from human β-hexosaminidase. J. biol. Chem.263 (1988) 4612–4618.
Mane, S. M., Marzella, L., Bainton, D. F., Holt, V. K., Cha, Y., Hildreth, J. E. K., and August, J. T., Purification and characterization of human lysosomal membrane glycoproteins. Archs Biochem. Biophys.268 (1989) 360–378.
Macquardt, T., Braulke, T., Hasilik, A., and von Figura, K., Association of the precursor of cathepsin D with coated membranes. Eur. J. Biochem.168 (1987) 37–42.
Mason, R. W., Green, G. D. J., and Barrett, A. J., Human liver cathepsin L. Biochem. J.226 (1985) 233–241.
Mayorga, L. S., Diaz, R., and Stahl, P. D., Reconstitution of endosomal proteolysis in a cell-free system. J. biol. Chem.264 (1989) 5392–5399.
Mechler, B., Hirsch, H. H., Müller, H., and Wolf, D. H., Biogenesis of the yeast lysosome (vacuole): biosynthesis and maturation of-proteinase yscB. EMBO J.7 (1988) 1705–1710.
Mechler, B., Müller, M., Meussdoerffer, F., and Wolf, D. H., In vivo biosynthesis of the vacuolar proteinases A and B in the yeastSaccharomyces cerevisiae. J. biol. Chem.257 (1982) 11203–11206.
Mechler, B., Müller, H., and Wolf, D. H., Maturation of vacuolar (lysosomal) enzymes in yeast: proteinase yscA and proteinase yscB are catalysts of the processing and activation event of carboxypeptidase yscY. EMBO J.6 (1987) 2157–2163.
Meloun, B., Baudys, M., Pohl, J., Pavlik, M., and Kostka, V., Amino acid sequence of bovine spleen cathepsin B. J. biol. Chem.263 (1988) 9087–9093.
Mierendorf, R. C. Jr, Cardelli, J. A., and Dimond, R. L., Pathways involved in targeting and secretion of a lysosomal enzyme in Dictyostelium discoideum. J. Cell Biol.100 (1985) 1777–1787.
Miller, A. L., Kress, B. C., Stein, R., Kinnon, C., Kern, H., Schneider, J. A., and Harms, E., Properties of N-acetyl-β-hexosaminidase from isolated normal and I-cell lysosomes. J. biol. Chem.256 (1981) 9352–9362.
Mizuochi, T., Nishimura, Y., Kato, K., and Kobata, A., Comparative studies of asparagine-linked oligosaccharide structures of rat liver microsomal and lysosomal β-glucuronidases. Archs Biochem. Biophys.209 (1981) 298–303.
Moehle, C. M., Dixon, C. K., and Jones, E. W., Processing pathway for protease B ofSaccharomyces cerevisiae. J. Cell Biol.108 (1989) 309–324.
Montreuil, J., Spatial conformation of glycans and glycoproteins. Biol. Cell.51 (1984) 115–131.
Morimoto, S., Yamamoto, Y., O'Brien, J. S., and Kishimoto, Y., Distribution of saposin proteins (sphingolipid activator proteins) in lysosomal storage and other diseases. Proc. natl Acad. Sci. USA87 (1990) 3493–3497.
Moriyama, A., Kageyama, T., and Takahashi, K., Identification of monkey lung procathepsin D-II as a pepsinogen-C-like acid protease zymogen. Eur. J. Biochem.132 (1983) 687–692.
Mort, J. S., and Recklies, A. D., Interrelationship of active and latent secreted human cathepsin B precursors. Biochem. J.233 (1986) 57–63.
Mortimore, G. E., and Ward, W. F., Internalization of cytoplasmic protein by hepatic lysosomes in basal and deprivation-induced proteolytic states. J. biol. Chem.256 (1981) 7659–7665.
Müller, M., and Müller, H., Synthesis and processing of in vitro and in vivo precursors of the vacuolar yeast enzyme carboxypeptidase Y. J. biol. Chem.256 (1981) 11962–11965.
Murray, G. J., Jonsson, L. V., Sorrell, S. H., Ginns, E. I., Tager, J. M., Schram A. W., and Barranger, J. A., Phosphorylation of β-glucocerebrosidase in cultured human fibroblasts. Fedn Proc.44 (1985) 709, abstr.
Mutsaers, J. H. G. M., Van Halbeek, H., Vliegenthart, J. F. G., Tager, J. M., Reuser, A. J. J., Kroos, M., and Galjaard, H., Determination of the structure of the carbohydrate chains of acid α-glucosidase from human placenta. Biochim. biophys. Acta911 (1987) 244–251.
Myerowitz, R., Piekarz, R., Neufeld, E. F., Shows, T. B., and Suzuki, K., Human β-hexosaminidase α chain: Coding sequence and homology with the β chain. Proc. natl Acad Sci. USA82 (1985) 7830–7834.
Nakao, Y., Kozutsumi, Y., Kawasaki, T., Yamashina, I., van Halbeek, H., and Vliegenthart, J. F. G., Oligosaccharides on cathepsin D from procine spleen. Archs Biochem. Biophys.229 (1984) 43–54.
Nanba, E., Tsuji, A., Omura, K., and Suzuki, Y., GM1-gangliosidosis: abnormalities in biosynthesis and early processing of β-galactosidase in fibroblasts. Biochem. biophys. Res. Commun.152 (1988) 794–800.
Nauseef, W. M., Myeloperoxidase biosynthesis by a human promyelocytic leukemia cell line: insight into myeloperoxidase deficiency. Blood67 (1986) 865–872.
Nauseef, W. M., Posttranslational processing of a human myeloid lysosomal protein, myeloperoxidase. Blood70 (1987) 1143–1150.
Neufeld, E. F., The uptake of enzymes into lysosomes: an overview. Birth Defects (March of Dimes Foundation)16 (1980) 77–84.
Neufeld, E. F., Natural history and inherited disorders of a lysosomal enzyme, β-hexosaminidase. J. biol. Chem.264 (1989) 10927–10930.
Neufeld, E. F., Lim, T. W., and Shapiro, L. J., Inherited disorders of lysosomal metabolism. A. Rev. Biochem.44 (1975) 357–376.
Nishimura, Y., Amano, J., Sato, H., Tsuji, H., and Kato, K., Biosynthesis of lysosomal cathepsins B and H in cultured rat hepatocytes. Archs Biochem. Biophys.262 (1988) 159–170.
Nishimura, Y., Furuno, K., and Kato, K., Biosynthesis and processing of lysosomal cathepsin L in primary cultures of rat hepatocytes. Archs Biochem. Biophys.263 (1988) 107–116.
Nishimura, Y., Higaki, M., and Kato, K., Identification of a precursor form of cathepsin D in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Biochem. biophys. Res. Commun.148 (1987) 335–343.
Nishimura, Y., and Kato, K., In vitro biosynthesis of the lysosomal cathepsin H. Biochem. biophys. Res. Commun.146 (1987) 159–164.
Nishimura, Y., and Kato, K., Intracellular transport and processing of lysosomal cathepsin B. Biochem. biophys. Res. Commun.148 (1987) 254–259.
Nishimura, Y., and Kato, K., Intracellular transport and processing of lysosomal cathepsin H. Biochem. biophys. Res. Commun.148 (1987) 329–334.
Nishimura, Y., and Kato, K., Identification of latent procathepsin H in microsomal lumen: characterization of proteolytic processing and enzyme activation. Archs Biochem. Biophys.260 (1988) 712–718.
Nishimura, Y., Kawabata, T., Furuno, K., and Kato, K., Evidence that aspartic proteinase is involved in the proteolytic processing event of procathepsin L in lysosomes. Archs Biochem. biophys.271 (1989) 400–406.
Nishimura, Y., Kawabata, T., and Kato, K., Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Archs Biochem. Biophys.261 (1988) 64–71.
Nishimura, Y., Kawabata, T., Yano, S., and Kato, K., Inhibition of intracellular sorting and processing of lysosomal cathepsin H and L at reduced temperature in primary cultures of rat hepatocytes. Archs Biochem. Biophys.283 (1990) 458–463.
Nishimura, Y., Kawabata, T., Yano, S., and Kato, K., Intracellular processing and activation of lysosomal cathepsins. Acta histochem. cytochem.23 (1990) 53–64.
Nishimura, Y., Rosenfeld, M. G., Kreibich, G., Gubler, U., Sabatini, D. D., Adesnik, M., and Andy, R., Nucleotide sequence of rat preputial gland β-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc. natl Acad. Sci. USA83 (1986) 7292–7296.
Novikoff, A. B., Lysosomes: a personal account, in: Lysosomes and Storage Diseases, pp. 1–41. Eds H. G. Hers and F. van Hoof. Academic Press, New York 1973.
O'Brien, J. S., and Kishimoto, Y., Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J.5 (1991) 301–308.
O'Brien, J. S., Kretz, K. A., Dewji, N., Wenger, D. A., Esch, F., and Fluharty, A. L., Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science241 (1988) 1098–1101.
O'Brien, J. S., de Wet, J., Fukushima, H., Wilcox, E., Dewji, N., McGee, J., Warner, T., Yoshida, A., Fluharty, A., Hill, F., and Helinski, D., Cloning of lysosomal genes, in: Molecular Basis of Lysosomal Storage Disorders, pp. 387–403. Eds J. A. Barranger and R. O. Brady. Academic Press, Orlando 1984.
Occhiodoro, T., Beckmann, K. R., Morris, C. P., and Hopwood, J. J., Human α-L-fucosidase: Complete coding sequence from cDNA clones. Biochem. biophys. Res. Commun.164 (1989) 439–445.
O'Dowd, B. F., Cumming, D. A., Gravel, R. A., and Mahuran, D., Oligosaccharide structure and amino acid sequence of the major glycopeptides of mature human β-hexosaminidase. Biochemistry27 (1988) 5216–5226.
O'Dowd, B. F., Quan, F., Willard, H. F., Lamhonwah A-M., Korneluk, R. G., Lowden, J. A., Gravel, R. A., and Mahuran, D. J., Isolation of cDNA clones coding for the β subunit of human β-hexosaminidase. Proc. natl. Acad. Sci. USA82 (1985) 1184–1188.
Ohsumi, Y., and Lee, Y. C., Mannose-receptor ligands stimulate secretion of lysosomal enzymes from rabbit alveolar macrophages. J. biol. Chem.262 (1987) 7955–7962.
Olsen, I., Abraham, D., Shelton, I., Bou-Gharios G., Muir, H., and Winchester, B., Cell contact induces the synthesis of a lysosomal enzyme precursor in lymphocytes and its direct transfer to fibroblasts. Biochim. biophys. Acta968 (1988) 312–322.
Olsen, I., Bou-Gharios, G., and Abraham, D., The activation of resting lymphocytes is accompanied by the biogenesis of lysosomal organelles. Eur. J. Immun.20 (1990) 2161–2170.
Olsson, I., Persson, A-M., and Strömberg, K., Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem. J.223 (1984) 911–920.
Orlacchio, A., Maffei, C., Beccari, T., and Lisi, P., β-N-acetyl-D-glucosaminidase isoenzymes by chromatofocusing from serum and skin in diabetes. Clinica chim. Acta141 (1984) 127–133.
Oshima, A., Kyle, J. W., Miller, R. D., Hoffmann, J. W., Powell, P. P., Grubb, J. H., Sly, W. S., Tropak, M., Guise, K. S., and Gravel, R. A., Cloning, sequencing, and expression of cDNA for human β-glucuronidase. Proc. natl Acad. Sci. USA84 (1987) 685–689.
Oude Elferink, R. P. J., van Doorn-van Wakeren, J., Hendriks, T., Strijland, A., and Tager, J. M., Transport and processing of endocytosed lysosomal α-glucosidase in cultured human skin fibroblasts. Eur. J. Biochem.158 (1986) 339–344.
Overdijk, B., Beem, E. P., Van Steijn, G. J., Trippelvitz, L. A. W., Lisman, J. J. W., Paz Parente, J., Cardon, P., Leroy, Y., Fournet, B., Van Halbeek, H., Mutsaers, J. H. G. M., and Vliegenthart, J. F. G., Structural analysis of the carbohydrate chains of β-N-acetylhexosaninidases from bovine brain. Biochem. J.232 (1985) 637–641.
Pannell, R., Wood, L., and Kaplan, A., Processing and secretion of α-mannosidase forms by Dictyostelium discoideum. J. biol. Chem.257 (1982) 9861–9865.
Patthy, L., Homology of the precursor of pulmonary surfactant —associated protein SP-B with prosaposin and sulfated glycoprotein 1. J. biol. Chem.266 (1991) 6035–6037.
Pelham, H. R. B., Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J.7 (1988) 913–918.
Pelham, H. R. B., The retention signal for soluble proteins of the endoplasmic reticulum. Trends biochem. Sci.15 (1990) 483–486.
Peters, C., Braun, M., Weber, B., Wendland, M., Schmidt, B., Pohlman, R., Waheed, A., and von Figura, K., Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J.9 (1990) 3497–3506.
Peters, P. J., Geuze, H. J., van der Donk, H. A., Slot, J. W., Griffith, J. M., Stam, N. J., Clevers, H. C., and Borst, J., Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur. J. Immun.19 (1989) 1469–1475.
Pfanner, N., and Neupert, W., The mitochondrial protein import apparatus. A. Rev. Biochem.59 (1990) 331–353.
Pfanner, N., Söllner, T., and Neupert, W., Mitochondrial import receptors for precursor proteins. Trends biochem. Sci.16 (1991) 63–67.
Pfeffer, S. R., Mannose 6-phosphate receptors and their role in targeting proteins to lysosomes. J. Membr. Biol.103 (1988) 7–16.
Pfeffer, S. R., and Rothman, J. E., Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. A. Rev. Biochem.56 (1987) 829–852.
Pohlmann, R., Krentler, C., Schmidt, B., Schröder, W., Lorkowski, G., Cully, J., Mersmann, G., Geier, C., Waheed, A., Gottschalk, S., Grzeschik, K-H., Hasilik, A., and von Figura, K., Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J.7 (1988) 2343–2350.
Pohlmann, R., Krüger, S., Hasilik, A., and von Figura, K., Effect of monensin on intracellular transport and receptor-mediated endocytosis of lysosomal enzymes. Biochem. J.217 (1984) 649–658.
Portnoy, D. A., Erickson, A. H., Kochan, J., Ravetch, J. V., and Unkeless, J. C., Cloning and characterization of a mouse cysteine proteinase. J. biol. Chem.261 (1986) 14697–14703.
Potier, M., Lamontagne, S., Michaud, L., and Tranchemontagne, J., Human neuraminidase is a 60 kDa-processing product of prosaposin. Biochem. biophys. Res. Commun.173 (1990) 449–456.
Potier, M., Michaud, L., Tranchemontagne, J., and Thauvette, L., Structure of the lysosomal neuraminidase-β-galactosidase-carboxypeptidase multienzymic complex. Biochem. J.267 (1990) 197–202.
Pott, G., Schneider, M., and Gerlach, U., Demonstration of isoenzymes of N-acetyl-beta-glucosaminidase by electrofocusing in thin layers of polyacrylamide gel. Sci. Tools25 (1978) 67–69.
Powell, P. P., Kyle, J. W., Miller, R. D., Pantano, J., Grubb, J. H., and Sly, W. S., Rat liver β-glucuronidase. Biochem. J.250 (1988) 547–555.
Proia, R. L., Gene encoding the human β-hexosaminidase β chain: Extensive homology of intron placement in the α- and β-chain genes. Proc. natl Acad. Sci. USA85 (1988) 1883–1887.
Proia, R. L., and Neufeld, E. F., Synthesis of β-hexosaminidase in cell-free translation and in intact fibroblasts: An insoluble precursor α chain in a rare form of Tay-Sachs disease. Proc. natl Acad. Sci. USA79 (1982) 6360–6364.
Proia, R. L., and Soravia, E., Organization of the gene encoding the human β-hexosaminidase α-chain. J. biol. Chem.262 (1987) 5677–5681.
Puizdar, V., and Turk, V., Cathepsinogen D: Characterization and activation to cathepsin D and inhibitory peptides. FEBS Lett.132 (1981) 299–304.
Quon, D. V. K., Proia, R. L., Fowler, A. V., Bleibaum, J., and Neufeld, E. F., Proteolytic processing of the β-subunit of the lysosomal enzyme, β-hexosaminidase, in normal human fibroblasts. J. biol. Chem.264 (1989) 3380–3384.
Radons, J., Isidoro, C., and Hasilik, A., Brefeldin A prevents uncovering but not phosphorylation of the recognition marker in cathepsin D. Biol. Chem. Hoppe-Seyler371 (1990) 567–573.
Rapoport, T. A., Protein transport across the ER membrane. Trends biochem. Sci.15 (1990) 355–358.
Reilly, J. J. Jr, Mason, R. W., Chen P., Joseph, L. J., Sukhatme, V. O., Yee, R., and Chapman, H. A. Jr., Synthesis and processing of cathepsin L, an clastase, by human alveolar macrophages. Biochem. J.257 (1989) 493–498.
Reiner, O., Dagan, O., and Horowitz, M., Human sphingolipid activator protein-1 and sphingolipid activator protein-2 are encoded by the same gene. J. molec. Neurosci.1 (1989) 225–233.
Reuser, A. J. J., Kroos, M., Oude Elferink, R. P. J., and Tager, J. M., Defects in synthesis, phosphorylation, and maturation of acid α-glucosidase in glycogenosis type II. J. biol. Chem.260 (1985) 8336–8341.
Riches, D. W. H., and Stanworth, D. R., Primary amines induce selective release of lysosomal enzymes from mouse macrophages. Biochem. J.188 (1980) 933–936.
Ritonja, A., Popovic, T., Kotnik, M., Machleidt, W., and Turk, V., Amino acid sequences of the human kidney cathepsins H and L. FEBS Lett.228 (1988) 341–345.
Robinson, J. S., Klionsky, D. J., Banta, L. M., and Emr, S. D., Protein sorting in Saccharomyces cerevisiae: Isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Molec. cell. Biol.8 (1988) 4936–4948.
Rose, J. K., and Doms, R. W., Regulation of protein export from the endoplasmic reticulum. A. Rev. Cytol.4 (1988) 258–288.
Rosenfeld, M. G., Kreibich, G., Popov, D., Kato, K., and Sabatini, D. D., Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J. Cell Biol.93 (1982) 135–143.
Rothman, J. H., Hunter, C. P., Valls, L. A., and Stevens, T. H., Overproduction-induced mislocalization of a yeast vacuolar protein allows isolation of its structural gene. Proc. natl Acad. Sci. USA83 (1986) 3248–3252.
Rothman, J. H., and Stevens, T. H., Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell47 (1986) 1041–1051.
Rothman, J. H., Yamashiro, C. T., Kane, P. M., and Stevens, T. H., Protein targeting to the yeast vacuole. Trends biochem. Sci.14 (1989) 347–350.
Saheki, T., and Holzer, H., Proteolytic activities in yeast. Biochim. biophys. Acta384 (1975) 203–214.
Salminen, A., and Gottesman, M. M., Inhibitor studies indicate that active cathepsin L is probably essential to its own processing in cultured fibroblasts. Biochem. J.272 (1990) 39–44.
Samarel, A. M., Ferguson, A. G. Decker, R. S., and Lesch, M., Effects of cysteine protease inhibitors on rabbit cathepsin D maturation. Am. J. Physiol.257 (1989) C1069–C1079.
Samarel, A. M., Worobec, S. W., Ferguson, A. G., Decker, R. S., and Lesch, M., Limited proteolysis of rabbit cardiac procathepsin D in a cell-free system. Am J. Physiol.250 (1986) C589–C596.
San Segundo, B., Chan, S. J., and Steiner, D. F., Identification of cDNA clones encoding a precursor of rat liver cathepsin B. Proc. natl Acad. Sci. USA82 (1985) 2320–2324.
Sandhoff, K., and Conzelmann, E., The biochemical basis of gangliosidoses. Neuropediatrics15 (1985) suppl. 85–92.
Sandoval, I. V., Chen, J. W., Yuan, L., and August, J. T., Lysosomal integral membrane glycoproteins are expressed at high levels in the inclusion bodies of I-cell disease fibroblasts. Archs Biochem. Biophys.271 (1989) 157–167.
Sano, A., Radin, N. S., Johnson, L. L., and Tarr, G. E., The activator protein for glucosylceramide β-glucosidase from Guinea pig liver. J. biol. Chem.263 (1988) 19597–19601.
Schäfer, W., Kalisz, H., and Holzer, H., Evidence for nonvacuolar proteolytic catabolite inactivation of yeast fructose-1,6-bisphosphatase. Biochim. biophys. Acta925 (1987) 150–155.
Schlesinger, P. H., Rodman, J. S., Doebber, T. W., Stahl, P. D., Lee, Y. C., Stowell, C. P., Kuhlenschmidt, T. B., The role of extra-hepatic tissues in the receptor-mediated plasma clearance of glycoproteins terminated by mannose or N-acetylglucosamine. Biochem. J.192 (1980) 597–606.
Schnyder, J., and Baggiolini, M., Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J. exp. Med.148 (1978) 435–450.
Schönholzer, F., Schweingruber, A-M. Trachsel, H., and Schweingruber, M. W., Intracellular maturation and secretion of acid phosphatase ofSaccharomyces cerevisiae. Eur. J. Biochem.147 (1985) 273–279.
Schröder, M., Klima, H., Nakano, T., Kwon, H., Quintern, L.E., Gärtner, S., Suzuki, K., and Sandhoff, K., Isolation of a cDNA encoding the human GM2 activator protein. FEBS Lett.251 (1989) 197–200.
Schorlemmer, H. U., Davies, P., Hylton, W., Gugig, M., and Allison, A. C., The selective release of lysosomal acid hydrolases from mouse peritoneal macrophages by stimuli of chronic inflammation. Br. J. exp. Path.58 (1977) 315–326.
Scriver, C. R., Beaudet, A. L., Sly, W. S., and Valle, D. (Eds), The Metabolic Basis of Inherited Disease, 6th edn., vol. 1, pp. 1–1475 and vol. 2, pp. 1477–3006. McGraw-Hill, New York 1989.
Shepherd, V. L., Konish, M. G., and Stahl, P., Dexamethasone increases expression of mannose receptors and decreases extracellular lysosomal enzyme accumulation in macrophages. J. biol. Chem.260 (1985) 160–164.
Shewale, J. G., Takahashi, T., and Tang, J., The primary structure of cathepsin D and the implications for its biological functions, in: Aspartic Proteinases and Their Inhibitors, pp. 101–116. Ed. V. Kostka. Walter de Gruyter & Co, Berlin 1985.
Shewale, J. G., and Tang, J., Amino acid sequence of porcine spleen cathepsin D. Proc. natl Acad. Sci. USA81 (1984) 3703–3707.
Silva, I. A., Murrills, R. J., and Etherington, D. J., Microelectrodes studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell. Res.5 (1988) 266–276.
Skudlarek, M. D., Novak, E. K., and Swank, R. T., Processing of lysosomal enzymes in macrophages and kidney, in: Lysosomes in Biology and Pathology, pp. 17–43. Eds J. T. Dingle, R. T. Dean and W. Sly. Elsevier Sci. Publ., Amsterdam 1984.
Skudlarek, M. D., and Swank, R. T., Biosynthesis of two lysosomal enzymes in macrophages. J. biol. Chem.254 (1979) 9939–9942
Skudlarek, M. D., and Swank, R. T., Turnover of two lysosomal enzymes in macrophages. J. biol. Chem.256 (1981) 10137–10144.
Slot, L. A., and Hendil, K. B., α2-Macroglobulin used to isolate intracellular endopeptidases from mammalian cells in culture. Biochem. J.255 (1988) 437–443.
Sly, W. S., and Fischer, H. D., The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J. Cell Biochem.18 (1982) 67–85.
Smith, R. J., Wierenga, W., and Iden, S. S., Characteristics of N-formyl-methionyl-leucyl-phenylalanine as an inducer of lysosomal enzyme release from human neutrophils. Inflammation4 (1980) 73–88.
Sonderfeld-Fresko, S., and Proia, R. L., Synthesis and assembly of a catalytically active lysosomal enzyme β-hexosaminidase B, in a cell-free system. J. biol. Chem.263 (1988) 13463–13469.
Sonderfeld-Fresko, S., and Proia, R. L., Analysis of the glycosylation and phosphorylation of the lysosomal enzyme, β-hexosaminidase B, by site-directed mutagenesis. J. biol. Chem.264 (1989) 7692–7697.
Sorge, J., West, C., Westwood, B., and Beutler, E., Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc. natl Acad. Sci USA82 (1985) 7289–7293.
Stahl, P. D., Rodman, J. S., Miller, M. J., and Schlesinger, P. H., Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc. natl Acad. Sci. USA75 (1978) 1399–1403.
Stahl, P. D., Wileman, T. E., and Shepherd, V. L., The mannose recognition pathway-implications for lysosome physiology, in: Molecular Basis of Lysosomal Storage Disorders, pp 209–218. Eds J. A. Barranger and R. O. Brady. Academic Press, Inc., Orlando 1984.
Starkey, P. M., and Barrett, A. J., Human cathepsin B1. Inhibition by α2-macroglobulin and other serum proteins. Biochem. J.131 (1973) 823–831.
Steckel, F., Hasilik, A., and von Figura, K., Multiple sulfatase deficiency: Degradation of arylsulfatase A and B after endocytosis in fibroblasts. Eur. J. Biochem.151 (1985) 147–152.
Stein, C., Gieselman, V., Kreysing, J., Schmidt, B., Pohlmann, R., Waheed, A., Meyer, H. E., O'Brien, J. S., and von Figura, K., Cloning and expression of human arylsulfatase A. J. biol. Chem.264 (1989) 1252–1259.
Stein, C., Hille, A., Seidel, J., Rijnbout, S., Waheed, A., Schmidt, B., Geuze, H., and von Figura, K., Cloning and expression of human steroid-sulfatase. J. biol. Chem.264 (1989) 13865–13872.
Stirling, J., Leung, A., Gravel, R. A., and Mahuran, D., Localization of the pro-sequence within the total deduced primary structure of human β-hexosaminidase B. FEBS Lett.231 (1988) 47–50.
Strömberg, K., Persson, A-M., and Olsson, I., The processing and intracellular transport of myeloperoxidase — modulation by lysosomotropic agents and monensin. Eur. J. Cell. Biol.39 (1986) 424–431.
Suzuki, Y., Sakuraba, H., Hayashi, K., Suzuki, K., and Imahori, K., β-galactosidase-neuraminidase deficiency: Restoration of β-galactosidase activity by protease inhibitors. J. Biochem. Tokyo90 (1981) 271–273.
Takahashi, T., Dehdarani, A. H., and Tang, J., Porcine spleen cathepsin H hydrolyzes oligopeptides solely by aminopeptidase activity. J. biol. Chem.263 (1988) 10952–10957.
Takahashi, T., Dehdarani, A. H., Yonezawa, S., and Tang, J., Porcine spleen cathepsin B is an exopeptidase. J. biol. Chem.261 (1986) 9375–9381.
Takahashi, T., Schmidt, P. G., and Tang, J., Oligosaccharide units of lysosomal cathepsin D from porcine spleen. J. biol. Chem.258 (1983) 2819–2830.
Takahashi, T., Schmidt, P. G., and Tang, J., Novel carbohydrate structures of cathepsin B from porcine spleen. J. biol. Chem.259 (1984) 6059–6062.
Takahashi, T., and Tang, J., Amino acid sequence of porcine spleen cathepsin D light chain. J. biol. Chem.258 (1983) 6435–6443.
Takahashi, T., Yonezawa, S., Dehdarani, A. H., and Tang, J., Comparative studies of two cathepsin B isozymes from porcine spleen. J. biol. Chem.261 (1986) 9368–9374.
Takasaki, S., Murray, G. J., Furbish, F. S., Brady, R. O., Barranger, J. A., and Kobata, A., Structure of the N-asparagine-linked oligosaccharide units of human placental β-glucocerebrosidase. J. biol. Chem.259 (1984) 10112–10117.
Takio, K., Towatari, T., Katunuma, N., Teller, D. C., and Titani, K., Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc. natl Acad. Sci. USA80 (1983) 3666–3670.
Tanaka, Y., Yano, S., Furuno, K., Ishikawa, T., Himeno, M., and Kato, K., Transport of acid phosphatase to lysosomes does not involve passage through the cell surface. Biochem. biophys. Res. Commun.170 (1990) 1067–1073.
Tanaka, Y., Yano, S., Okada, K., Ishikawa, T., Himeno, M., and Kato, K., Lysosomal acid phosphatase is transported via endosomes to lysosomes. Biochem. biophys. Res. Commun.166 (1990) 1176–1182.
Tang, J., and Wong, R. N. S., Evolution in the structure and function of aspartic proteases. J. Cell Biochem.33 (1987) 53–63.
Taniguchi, T., Mizuochi, T., Towatari, T., Katunuma, N., and Kobata, A., Structural studies on the carbohydrate moieties of rat liver cathepsins B and H. J. Biochem., Tokyo97 (1985) 973–976.
Taylor, K. L., Guzman, G. S., Pohl, J., and Kinkade, J. M.Jr, Distinct chromatographic forms of human hemi-myeloperoxidase obtained by reductive cleavage of the dimeric enzyme. J. biol. Chem.265 (1990) 15938–15946.
Thomas, D. J., Richards, A. D., and Kay, I., Inhibition of aspartic proteinases by α2-macroglogulin. Biochem. J.259 (1989) 905–907.
Townsend, A., and Bodmer, H., Antigen recognition by class I-restricted T lymphocytes. A. Rev. Immun.7 (1989) 601–624.
Tranchenontagne, J., Michaud, L., and Potier, M., Deficient lysosomal carboxypeptidase activity in galactosialidosis. Biochem. biophys. Res. Commun.168 (1990) 22–29.
Troyen, B. R., Ascherman, D., Atlas, D., and Gottesman, M. M., Cloning and expression of the gene for the major excreted protein of transformed mouse fibroblasts. J. biol. Chem.263 (1988) 254–261.
Tschopp, J., and Nabholz, M., Perforin-mediated target cell lysis by cytotoxic T lymphocytes. A. Rev. Immun.8 (1990) 279–320.
Tsuji, S., Choudary, P. V., Martin, B. M., Winfield, S., Barranger, J. A., and Ginns, E. I., Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J. biol. Chem.261 (1986) 50–53.
Tsuji, S., Yamauchi, T., Hiraiwa, M., Isobe, T., Okuyama, T., Sakimura, K., Takahashi, Y., Nishizawa, M., Uda, Y., and Miyatake, T., Mole ular cloning of a full-length cDNA for human α-N-acetylgalactosaminidase (α-galactosidase B). Biochem. biophys. Res. Commun.163 (1989) 1498–1504.
Tsukamoto, T., Miura, S., and Fujiki, Y., Restoration by a 35K membrane protein of peroxisome assembly in a peroxisome-deficient mammalian cell mutant. Nature350 (1991) 77–78.
Ullrich, K., Basner, R., Gieselmann, V., and von Figura, K., Recognition of human urine α-N-acetylglucosaminidase by rat hepatocytes. Biochem. J.180 (1979) 413–419.
Ullrich, K., Gieselmann, V., Mersmann, G., and von Figura, K., Endocytosis of lysosomal enzymes by non-parenchymal rat liver cells. Biochem. J.182 (1979) 329–335.
van der Horst, G. T. J., Galjart, N. J., d'Azzo, A., Galjaard, H., and Verheijen, F. W., Identification and in vitro reconstitution of lysosomal neuraminidase from human placenta. J. biol. Chem.264 (1989) 1317–1322.
van Diggelen, O. P., Hoogeveen, A. T., Smith, P. J., Reuser, A. J. J., and Galjaard, H., Enhanced proteolytic degradation of normal β-galactosidase in the lysosomal storage disease with combined β-galactosidase and neuraminidase deficiency. Biochim. biophys. Acta703 (1983) 69–76.
van Diggelen, O. P., Schram, A. W., Sinnott, M. L., Smith, P. J., Robinson, D., and Galjaard, H., Turnover of β-galactosidase in fibroblasts from patients with genetically different types of β-galactosidase deficiency. Biochem. J.200 (1981) 143–151.
Verner, K., and Schatz, G., Protein translocation across membranes. Science241 (1988) 1307–1313.
Vladutiu, G. D., Effect of the co-existence of galactosyl and phosphomannosyl residues on β-hexosaminidase on the processing and transport of the enzyme in mucolipidosis I fibroblasts. Biochim. biophys. Acta760 (1983) 363–370.
Vladutiu, G. D., and Rattazzi, M. C., Abnormal lysosomal hydrolases excreted by cultured fibroblasts in I-cell disease (mucolipidosis II). Biochem. biophys. Res. Commun.67 (1975) 956–964.
Vladutiu, G. D., and Rattazzi, M. C., Excretion-reuptake route of β-hexosaminidase in normal and I-cell disease cultured fibroblasts. J. clin. Invest.63 (1979) 595–601.
von Figura, K., and Hasilik, A., Lysosomal enzymes and their receptors. A. Rev. Biochem.55 (1986) 167–193.
von Figura, K., Hasilik, A., and Steckel, F., Lysosomal storage disorders caused by instability of the missing enzymes, in: Molecular Basis of Lysosomal Storage Disorders, pp. 133–143. Eds J. A. Barranger and R. O. Brady. Academic Press, Inc., Orlando 1984.
von Figura, K., Steckel, F., Conary, J., Hasilik, A., and Shaw, E., Heterogeneity in late-onset metachromatic leukodystrophy. Effect of inhibitors of cysteine proteinases. Am. J. hum. Genet.39 (1986) 371–382.
von Figura, K., Steckel, F., and Hasilik, A., Juvenile and adult metachromatic leukodystrophy: Partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc. natl Acad. Sci. USA80 (1983) 6066–6070.
Wada, K., Takai, T., and Tanabe, T., Amino acid sequence of chicken liver cathepsin L. Eur. J. Biochem.167 (1987) 13–18.
Waheed, A., Gottschalk, S., Hille, A., Krentler, C., Pohlmann, R., Braulke, T., Hauser, H., Geuze, H., and von Figura, K., Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J.7 (1988) 2351–2358.
Waheed, A., Hasilik, A., and von Figura, K., Synthesis and processing of arylsulfatase A in human skin fibroblasts. Hoppe-Seyler's Z. Physiol. Chem.363 (1982) 425–430.
Waheed, A., Hasilik, A., and von Figura, K., UDP-N-acetylglucosamine: lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. J. biol. Chem.257 (1982) 12322–12331.
Waheed, A., Steckel, F., Hasilik, A., and von Figura, K., Two allelic forms of human arylsulfatase A with different numbers of asparagine-linked oligosaccharides. Am. J. hum. Genet.35 (1983) 228–233.
Waheed, A., and van Etten, R. L., Phosphorylation and sulfation of arylsulfatase A accompanies biosynthesis of the enzyme in normal and carcinoma cell lines. Biochim. biophys. Acta847 (1985) 53–61.
Wang, A. M., Bishop, D. F., and Desnick, R. J., Human α-N-acetylgalactosaminidase-molecular cloning, nucleotide sequence, and expression of a full-length cDNA. J. biol. Chem.265 (1990) 21859–21866.
Watanabe, T., Watanabe, M., Ishii, Y., Matsuba, H., Kimura, S., Fujita, T., Kominami, E., Katunuma, N., and Uchiyama, Y., An immunocytochemical study on co-localization of cathepsin B and atrial natriuretic peptides in secretory granules of atrial myoendocrine cells of rat heart. J. Histochem. Cytoche.37 (1989) 347–351.
Weber, W., Kehrer, T., Gressner, A. M., Stuhlsatz, H. W., and Greilling, H., Changes in the catalytic activities of proteoglycan-degrading lysosomal enzymes in parenchymal and non-parenchymal liver cells and in serum during the development of experimental liver fibrosis. J. clin. Chem. clin. Biochem.21 (1983) 287–293.
Weissmann, G., Korchak, H. M., Perez, H. D., Smolen, J. E., Goldstein, I. M., and Hoffstein, S. T., The secretory code of the neutrophil. J. reticuloendoth Soc.26 (1979) 687–700.
Whiting, P. H., Ross, I. S., and Borthwick, L., Serum and urine N-acetyl-β-D-glucosaminidase in diabetics on diagnosis and subsequent treatment, and stable insulin dependent diabetics. Clinica chim. Acta92 (1979) 459–463.
Wiederanders, B., and Kirschke, H., The processing of cathepsin L precursor in vitro. Archs Biochem. Biophys.272 (1989) 516–521.
Wiesmann, U., Vassella, F., Herschkowitz, N., “I-cell” disease: leakage of lysosomal enzymes into extracellular fluids. N. Engl. J. Med.285 (1971) 1090–1091.
Willcox, P., and Ratray, S., Secretion and uptake of β-N-acetylglucosaminidase by fibroblasts. Biochim. biophys. Acta586 (1979) 442–452.
Williams, M. A., and Fukuda, M., Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J. Cell Biol.111 (1990) 955–966.
Wilson, P. J., Morris, C. P., Anson, D. S., Occhiodoro, T., Bielicki, J., Clements, P. R., and Hopwood, J. J., Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc. natl Acad. Sci. USA87 (1990) 8531–8535.
Woolford, C. A., Daniels, L. B., Park, F. J., Jones, E. W., van Arsdell, J. N., and Innis, M. A., The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation ofSaccharomyces cerevisiae vacuolar hydrolases. Molec. cell. Biol.6 (1986) 2500–2510.
Yamada, M., Myeloperoxidase precursors in human myeloid leukemia HL-60 cells. J. biol. Chem.257 (1982) 5980–5982.
Yen, P. H., Allen, E., Marsh, B., Mohandas, T., Wang, N., Taggart, R. T., and Shapiro, L. J., Cloning and expression of steroid sulfatase cDNA and the frequent occurrence of deletions in STS deficiency: implications for X-Y interchange. Cell49 (1987) 443–454.
Yonezawa, S., Takahashi, T., Ichinose, M., Miki, K., Tanaka, J., and Gasa, S., Structural studies of rat cathepsin E: Amino-terminal structure and carbohydrate units of mature enzyme. Biochem. biophys. Res. Commun.166 (1990) 1032–1038.
Yonezawa, S., Takahashi, T., Wang, X-j., Wong, R. N. S., Hartsuck, J. A., and Tang, J., Structures at the proteolytic processing region of cathepsin D. J. biol. Chem.263 (1988) 16504–16511.
Yoshihisa, T., and Anraku, Y., A novel pathway of import of α-mannosidase, a marker enzyme of vacuolar membrane, inSaccharomyces cerevisiae. J. biol. Chem.265 (1990) 22418–22425.
Youngdhal-Turner, P., Rosenberg, L. E., and Allen, R. H., Binding and uptake of transcobalamin II by human fibroblasts. J. clin. Invest.61 (1978) 133–141.
Zubenko, G. S., Park, F. J., and Jones, E. W., Mutations in PEP4 locus ofSaccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc. natl Acad. Sci. USA80 (1983) 510–514.
Zühlsdorf, M., Imort, M., Hasilik, A., and von Figura, K., Molecular forms of β-hexosaminidase and cathepsin D in serum and urine of healthy subjects and patients with elevated activity of lysosomal enzymes. Biochem. J.213 (1983) 733–740.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasilik, A. Theearly andlate processing of lysosomal enzymes: Proteolysis and compartmentation. Experientia 48, 130–151 (1992). https://doi.org/10.1007/BF01923507
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01923507