Abstract.
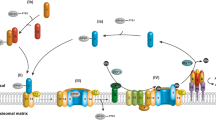
The peroxisomal protein import machinery displays remarkable properties. Be it its capacity to accept already folded proteins as substrates, its complex architecture or its energetics, almost every aspect of this machinery seems unique. The list of unusual properties is still growing as shown by the recent finding that one of its central components, Pex5p, is transiently monoubiquitinated at a cysteine residue. However, the data gathered in recent years also suggest that the peroxisomal import machinery is not that exclusive and similarities with p97/Cdc48-mediated processes and with multisubunit RING-E3 ligases are starting to emerge. Here, we discuss these data trying to distill the principles by which this complex machinery operates.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 16 July 2008; received after revision 25 August 2008; accepted 29 August 2008
Rights and permissions
About this article
Cite this article
Grou, C.P., Carvalho, A.F., Pinto, M.P. et al. The peroxisomal protein import machinery – a case report of transient ubiquitination with a new flavor. Cell. Mol. Life Sci. 66, 254–262 (2009). https://doi.org/10.1007/s00018-008-8415-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-008-8415-5