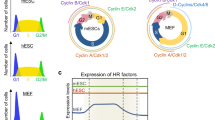
Abstract
Embryonic stem cells (ESCs) can undergo unlimited self-renewal and retain the pluripotency to differentiate into all cell types in the body. Therefore, as a renewable source of various functional cells in the human body, ESCs hold great promise for human cell therapy. During the rapid proliferation of ESCs in culture, DNA damage, such as DNA double-stranded breaks, will occur in ESCs. Therefore, to realize the potential of ESCs in human cell therapy, it is critical to understand the mechanisms how ESCs activate DNA damage response and DNA repair to maintain genomic stability, which is a prerequisite for their use in human therapy. In this context, it has been shown that ESCs harbor much fewer spontaneous mutations than somatic cells. Consistent with the finding that ESCs are genetically more stable than somatic cells, recent studies have indicated that ESCs can mount more robust DNA damage responses and DNA repair than somatic cells to ensure their genomic integrity.
Similar content being viewed by others
References
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Fu X, Xu Y (2012) Challenges to the clinical application of pluripotent stem cells: towards genomic and functional stability. Genome Med 4(6):55
Angelos MG, Kaufman DS (2015) Pluripotent stem cell applications for regenerative medicine. Curr Opin Organ Transplant 20(6):663–670
Ahuja AK, Jodkowska K, Teloni F, Bizard AH, Zellweger R, Herrador R, Ortega S, Hickson ID, Altmeyer M, Mendez J, Lopes M (2016) A short G1 phase imposes constitutive replication stress and fork remodelling in mouse embryonic stem cells. Nat Commun 7:10660. doi:10.1038/ncomms10660
Lamm N, Ben-David U, Golan-Lev T, Storchová Z, Benvenisty N, Kerem B (2016) Genomic instability in human pluripotent stem cells arises from replicative stress and chromosome condensation defects. Cell Stem Cell 18(2):253–261
Desmarais JA, Unger C, Damjanov I, Meuth M, Andrews P (2016) Apoptosis and failure of checkpoint kinase 1 activation in human induced pluripotent stem cells under replication stress. Stem Cell Res Ther 7(1):1–7
Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19(2):238–245
Iyama T, Wilson Iii DM (2013) DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 12(8):620–636
Tichy ED (2011) Mechanisms maintaining genomic integrity in embryonic stem cells and induced pluripotent stem cells. Exp Biol Med 236(9):987–996
Young Richard A (2011) Control of the embryonic stem cell state. Cell 144(6):940–954
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113(5):631–642
Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ (2005) High-efficiency RNA interference in human embryonic stem cells. Stem Cells 23(3):299–305
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113(5):643–655
Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T (2005) Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol Cell Biol 25(6):2475–2485
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P (2005) Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem 280(26):24731–24737
Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24(4):372–376
van den Berg DLC, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA (2010) An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell 6(4):369–381
Yuri S, Fujimura S, Nimura K, Takeda N, Toyooka Y, Fujimura Y-I, Aburatani H, Ura K, Koseki H, Niwa H, Nishinakamura R (2009) Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells 27(4):796–805
Wu Q, Chen X, Zhang J, Loh Y-H, Low T-Y, Zhang W, Zhang W, Sze S-K, Lim B, Ng H-H (2006) Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem 281(34):24090–24094
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Mills KD, Ferguson DO, Alt FW (2003) The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev 194:77–95
Mladenov E, Magin S, Soni A, Iliakis G (2016) DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: cell cycle and proliferation-dependent regulation. Semin Cancer Biol 37–38:51–64
Cremona CA, Behrens A (2014) ATM signalling and cancer. Oncogene 33(26):3351–3360
Sirbu BM, Cortez D (2013) DNA damage response: three levels of DNA repair regulation. Cold Spring Harb Perspect Biol 5(8):a012724
Liu D, Xu Y (2010) p53, oxidative stress, and aging. Antioxid Redox Signal 15(6):1669–1678
Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, Yoon CJ, Kim DW, Kim SH, Moon SY (2005) Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells 23(2):211–219
Cho YM, Kwon S, Pak YK, Seol HW, Choi YM, Park DJ, Park KS, Lee HK (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun 348(4):1472–1478
St. John JC, Ramalho-Santos J, Gray HL, Petrosko P, Rawe VY, Navara CS, Simerly CR, Schatten GP (2005) The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells 7(3):141–153
Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, John JCS (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120(22):4025–4034
John JCS, Amaral A, Bowles E, Oliveira JF, Lloyd R, Freitas M, Gray HL, Navara CS, Oliveira G, Schatten GP (2006) The analysis of mitochondria and mitochondrial DNA in human embryonic stem cells. In: Turksen K (ed) Human embryonic stem cell protocols. Springer, Berlin, pp 347–374
Xu X, Duan S, Yi F, Ocampo A, Liu G-H, Belmonte JCI (2013) Mitochondrial regulation in pluripotent stem cells. Cell Metab 18(3):325–332
Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 28(4):721–733
Maynard S, Swistowska AM, Lee JW, Liu Y, Liu S-T, Da Cruz AB, Rao M, de Souza-Pinto NC, Zeng X, Bohr VA (2008) Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells 26(9):2266–2274
Momcilovic O, Knobloch L, Fornsaglio J, Varum S, Easley C, Schatten G (2010) DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS One 5(10):e13410
Schneider L, Pellegatta S, Favaro R, Pisati F, Roncaglia P, Testa G, Nicolis SK, Finocchiaro G, di Fagagna FdA (2013) DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Rep 1(2):123–138
Tichy ED, Pillai R, Deng L, Liang L, Tischfield J, Schwemberger SJ, Babcock GF, Stambrook PJ (2010) Mouse embryonic stem cells, but not somatic cells, predominantly use homologous recombination to repair double-strand DNA breaks. Stem Cells Dev 19(11):1699–1711
Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee S-L, Stagg CA, Hoang HG, Yang H-T, Indig FE, Wersto RP, Ko MSH (2010) Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464:858–863
Zhao B, W-d Zhang, Duan Y-l Lu, Y-q Cun Y-x, C-h Li, Guo K, W-h Nie, Li L, Zhang R, Zheng P (2015) Filia is an ESC-specific regulator of DNA damage response and safeguards genomic stability. Cell Stem Cell 16(6):684–698
Xiong J, Todorova D, Su NY, Kim J, Lee PJ, Shen Z, Briggs SP, Xu Y (2015) Stemness factor Sall4 is required for DNA damage response in embryonic stem cells. J Cell Biol 208(5):513–520
Chuykin IA, Lianguzova MS, Pospelova TV, Pospelov VA (2008) Activation of DNA damage response signaling in mouse embryonic stem cells. Cell Cycle 7(18):2922–2928
Yong KJ, Gao C, Lim JSJ, Yan B, Yang H, Dimitrov T, Kawasaki A, Ong CW, Wong K-F, Lee S, Ravikumar S, Srivastava S, Tian X, Poon RT, Fan ST, Luk JM, Dan YY, Salto-Tellez M, Chai L, Tenen DG (2013) Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med 368(24):2266–2276
Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, Zhang X, Yang T, Cai J, Yan Y, Mao F, Zhu W, Shao Q, Qian H, Xu W (2014) SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene 33(48):5491–5500
Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3(3):155–168
Adams BR, Hawkins AJ, Povirk LF, Valerie K (2010) ATM-independent, high-fidelity nonhomologous end joining predominates in human embryonic stem cells. Aging (Albany NY) 2(9):582
Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM (1998) ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 8(3):145–155
Chao C, Saito S, Anderson CW, Appella E, Xu Y (2000) Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci USA 97(22):11936–11941
Hong Y, Stambrook PJ (2004) Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci USA 101(40):14443–14448
Dolezalova D, Mraz M, Barta T, Plevova K, Vinarsky V, Holubcova Z, Jaros J, Dvorak P, Pospisilova S, Hampl A (2012) MicroRNAs regulate p21Waf1/Cip1 protein expression and the DNA damage response in human embryonic stem cells. Stem Cells 30(7):1362–1372
Suvorova II, Grigorash BB, Chuykin IA, Pospelova TV, Pospelov VA (2016) G1 checkpoint is compromised in mouse ESCs due to functional uncoupling of p53-p21Waf1 signaling. Cell Cycle 15(1):52–63
Rocha CRR, Lerner LK, Okamoto OK, Marchetto MC, Menck CFM (2013) The role of DNA repair in the pluripotency and differentiation of human stem cells. Mutat Res 752(1):25–35
Huskey NE, Guo T, Evason KJ, Momcilovic O, Pardo D, Creasman KJ, Judson RL, Blelloch R, Oakes SA, Hebrok M, Goga A (2015) CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Rep 4(3):374–389
Dumitru R, Gama V, Fagan BM, Bower JJ, Swahari V, Pevny LH, Deshmukh M (2012) Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol Cell 46(5):573–583
Li M, He Y, Dubois W, Wu X, Shi J, Huang J (2012) Distinct regulatory mechanisms and functions for p53-activated and p53-repressed DNA damage response genes in embryonic stem cells. Mol Cell 46(1):30–42
Liu JC, Guan X, Ryan JA, Rivera AG, Mock C, Agarwal V, Letai A, Lerou PH, Lahav G (2013) High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell 13(4):483–491
Harris SL, Levine AJ (2005) The p53 pathway: positive and negative feedback loops. Oncogene 24(17):2899–2908
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10(4):431–442
Friborg J Jr, Kong W, Hottiger MO, Nabel GJ (1999) p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889–894
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431
Song H, Chung S-K, Xu Y (2010) Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell 6(1):80–89
Chao C, Saito S, Kang J, Anderson CW, Appella E, Xu Y (2000) p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J 19(18):4967–4975
Grandela C, Pera MF, Wolvetang EJ (2008) p53 is required for etoposide-induced apoptosis of human embryonic stem cells. Stem Cell Res 1(2):116–128
Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y (2005) p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7(2):165–171
Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T, Suemori H, Nakatsuji N, Tada T (2005) Pluripotential competence of cells associated with Nanog activity. Mech Dev 122(1):67–79
Jain AK, Allton K, Iacovino M, Mahen E, Milczarek RJ, Zwaka TP, Kyba M, Craig Barton M (2012) p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol 10(2):424
Zhao T, Xu Y (2010) p53 and stem cells: new developments and new concerns. Trends Cell Biol 20(3):170–175
Vaziri H, Dessain SK, Eaton EN, Imai S-I, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107(2):149–159
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107(2):137–148
Han M-K, Song E-K, Guo Y, Ou X, Mantel C, Broxmeyer HE (2008) SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2(3):241–251
Zhang Z-N, Chung S-K, Xu Z, Xu Y (2014) Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells 32(1):157–165
Acknowledgments
This study is supported by a grant from the National High-tech R&D Program (863 Program No. 2015AA020310), Guangzhou Key Laboratory of Tumor Immunology Research, Guangdong Province Key Special Science and Technology Project (2015B020225004), South Wisdom Valley Innovative Research Team Program (2014) No. 365, Shenzhen Municipal Science and Technology Innovation Council (20140405201035), and the National Natural Science Foundation of China (Nos. 815300045, 81373166, and 81430032).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fu, X., Cui, K., Yi, Q. et al. DNA repair mechanisms in embryonic stem cells. Cell. Mol. Life Sci. 74, 487–493 (2017). https://doi.org/10.1007/s00018-016-2358-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2358-z